 |
 |
AbstractPurposeTo determine the incidence, risk factors, and clinical characteristics of pelvic insufficiency fracture (PIF) in patients with cervical cancer.
Materials and MethodsBetween July 2004 and August 2009, 235 patients with non-metastatic cervical cancer were treated with definitive chemoradiation or postoperative radiotherapy. Among 235 patients, 117 (49.8%) underwent the first positron emission tomography/computed tomography (PET/CT) within 1 year after radiotherapy. The median radiation dose was 55 Gy (range, 45 to 60 Gy). Medical charts and imaging studies, including PET/CT, magnetic resonance imaging (MRI), CT, bone scintigraphy were reviewed to evaluate the patients with PIF.
ResultsAmong 235 patients, 16 developed PIF. The 5-year detection rate of PIF was 9.5%. The 5-year detection rate of PIF in patients who underwent the first PET/CT within a year was 15.6%. The median time to development of PIF was 12.5 months (range, 5 to 30 months). The sites of fracture included 12 sacroiliac joints, 3 pubic rami, 3 iliac bones, and 1 femoral neck. Eleven of 16 patients having PIF complained of hip pain requiring medications. One patient required hospitalization for pain control. The significant risk factors of PIF were old age, body mass index less than 23, bone mineral density less than -3.5 SD, and the first PET/CT within a year after radiotherapy. Radiation dose and concurrent chemotherapy had no impact on PIF rate.
IntroductionInsufficiency fracture is a kind of stress fracture, which is caused by normal stress placed on weakened bone. Bone demineralization and decreased bone elastic resistance result from osteoporosis, previous radiotherapy, rheumatoid arthritis, prolonged corticosteroid therapy, and renal failure [1]. Although they are rarely life-threatening, these fractures deserve special attention with respect to quality of life of each patient [2,3].
Although pelvic insufficiency fracture (PIF) after pelvic radiotherapy has been regarded as a rare complication, recent studies reported the incidence of PIF to be as high as 8.2 to 19.7%, not only in gynecologic patients but also in rectal, prostate, and anal cancer patients [2,4-8]. These fractures, in particular sacral fractures, tend to be relatively underdiagnosed [9-13]. Although PIF can cause symptoms like pelvic pain, in some cases, there may be only mild or no symptoms.
In recent years, positron emission tomography/computed tomography (PET/CT) is increasingly used as a follow-up tool for patients with cervical cancer at our institution. Several studies reported an increased fluorodeoxyglucose (FDG) uptake by PIF on PET/CT [14-17]. Although it is now not a rare situation that we diagnose PIF through PET/CT, there has been no reports that have estimated the incidence of PIF including cases diagnosed with PET/CT. In this study, we report our experiences of 11 cases of symptomatic PIF and 5 cases of asymptomatic PIF after pelvic radiotherapy in cervical cancer patients. We compared the incidence of PIF before and after the introduction of PET/CT, then evaluated the risk factors of PIF.
Materials and Methods1. Patient selectionBetween July 2004 and October 2009, 235 newly diagnosed cervical cancer patients without evidence of distant metastasis received curative radiotherapy or postoperative radiotherapy with/without concurrent chemotherapy at our institution. Medical records including radiation oncology, gynecology, and orthopedics of these patients were reviewed for analysis.
2. Patient characteristics and treatmentsThe median age was 55 years (range, 23 to 86 years). The median body mass index (BMI) value was 22.8 (range, 15.8 to 34.5). The International Federation of Gynecology and Obstetrics (FIGO) stage was I in 43 (18.3%), II in 141 (60.0%), III in 35 (14.9%), and IVA in 16 (6.8%). Fifty-one patients (21.7%) received surgery before radiotherapy, 184 (78.3%) were treated with definitive radiotherapy, 192 (81.7%) received concurrent chemoradiotherapy. The median follow-up time was 24 months (range, 5 to 72 months). Table 1 shows patient characteristics according to performance of the first PET/CT within a year after radiotherapy.
External beam radiation therapy was delivered in 1.8 Gyfractions, Monday through Friday, over 5-7 weeks, using 10 MV photons with customized blocking. The median dose of radiotherapy was 55 Gy (range, 45 to 60 Gy). A four-field box technique was used. The general whole-pelvic fields prescribed were as follows. The superior border was the L4-L5 vertebral level. The inferior border was at the bottom of the obturator foramen or 2-3 cm below the lowest extent of the cervical or vaginal disease. The lateral borders were placed 2 cm lateral to the inner bony margins of the true pelvis. For the lateral fields, the anterior border included symphysis pubis, and the posterior border was the S2-3 interspace.
Patients were scheduled for follow-up visits every 3 to 4 months for the first 2 years, every 6 months for up to 5 years, and annually thereafter. Before 2006, we performed pelvic CT and/or pelvic magnetic resonance imaging (MRI) for monitoring cervical cancer patients. Since 2006, we adopted PET/CT for monitoring cervical cancer patients within a year after completion of radiotherapy. Among 235 patients, 117 (49.8%) underwent the first PET/CT within a year after pelvic radiotherapy. Twenty-two (9.4%) patients underwent MRI after pelvic radiotherapy. Nine patients (3.8%) underwent bone scintigraphy after pelvic radiotherapy.
3. Diagnostic criteria of PIFAll imaging studies and clinical follow-up notes were carefully reviewed for 235 patients to identify those with possible fractures in their pelvic sites in the radiation fields. Contrast-enhanced pelvic CT or PET/CT were obtained during surveillance. Patients who complained pelvic pain underwent bone scintigraphy, pelvic MRI, or bone densitometry additionally. The diagnostic criteria for PIF were as follows; 1) the classic "H" pattern on bone scintigraphy was regarded as diagnostic. In case of regionally increased tracer uptake was present but the "H" pattern was absent, additional imaging studies or workups for diagnosis were needed, 2) demonstration of fracture lines or increased sclerosis without osteolytic lesion on CT scanning was regarded as diagnostic, 3) the findings of decreased signal on T1 and increased signal on T2 without soft tissue mass on MRI, 4) diffuse and mild FDG uptake (maximum standard uptake value less than 5.6) [15,17-20] of pelvic bone or sacrum were regarded as diagnostic on PET/CT.
4. Statistical analysisWe calculated the actuarial incidence of PIF by Kaplan-Meier method. Risk factors that could affect the incidence of PIF such as age, BMI, bone mineral density (BMD), concurrent chemotherapy, the first PET/CT within a year after radiotherapy, radiation dose, and surgery were assessed by log-rank test. The difference of patient characteristics was compared by chi-square test and independent sample t-test. Statistical analyses were performed with SPSS ver. 18.0 (IBM, New York, NY, USA).
Results1. IncidenceAmong 235 patients, 16 (6.8%) developed PIF. The estimated 5-year detection rate of PIF was 9.5%. The median time to detection of PIF was 12.5 months (range, 5 to 30 months) (Fig. 1).
2. SitesThe PIF were located at sacroiliac joint (12 sites), pubic ramus (3 sites), femoral neck (1 site), iliac wing (1 site), and acetabulum (2 sites). One patient developed fracture of the femoral neck which had been included in the lateral radiation portal. Two patients had multiple fracture sites (Table 2).
3. Analysis of risk factorsOn univariate analysis, the risk of PIF was significantly higher in women who were older than 75 years (p = 0.02), who had a BMI value less than 23 (p = 0.04), who had a BMD score less than -3.5 SD (p = 0.02), and who underwent the first PET/CT within a year after the completion of radiotherapy (p = 0.02) (Table 3). The risk of PIF was not significantly associated with surgery, chemotherapy, or radiotherapy dose. The analysis of factors such as rheumatoid arthritis, chronic renal failure, and diabetes was not available because of the small incidence in cervical cancer patients. Multivariate analysis was not done because BMI and BMD variables had high numbers of missing data.
4. Clinical courseAmong 16 PIF cases, 11 had symptoms requiring medications and 5 were asymptomatic. All the symptomatic patients were improved with medications except one who visited emergency room and required hospitalization for pain control. None required operative procedures or other interventional procedures.
5. Serial PET/CT findingsAmong 16 PIF cases, 7 have been followed up by PET/CT serially (Table 4). Abnormal FDG uptakes on bone were normalized in 4 patients (Fig. 2), whereas 2 patients showed decreased but persistent uptakes. In one patient, follow-up PET/CT showed a new FDG-uptake lesion just near the PIF site, though the original PIF site was normalized (patient 3) (Fig. 3). In one patient who had decreased but persistent uptake on the 12-month follow-up PET/CT, abnormal uptake on bone was normalized on the 16-month follow-up PET/CT (patient 10).
Discussion and ConclusionPIF had been regarded as a rare complication after radiotherapy. But recent studies showed that PIFs after pelvic radiotherapy were not rare. They reported the actuarial incidence of PIF at 5 years as 8.2 to 19.7% [2,4-6]. In our series, the overall incidence of PIF at 5 years was relatively low (9.5%) when compared to those series. In subgroup analysis, however, the detection rate of PET/CT group (15.6%) was comparable to those series. In the subgroup in which PET/CT was not done early, the detection rate of PIF was quite low (3.4%), which was thought to be the result of low prescription rate of MRI or bone scintigraphy at our institution. There is also a possibility that asymptomatic cases were unnoticed and not evaluated properly.
Until recently PIF has been diagnosed by plain radiograph, bone scintigraphy, CT, and MRI. Plain radiograph of the pelvis can show sclerotic bands, cortical destruction, and fracture line in the sacral ala. These findings, however, are too subtle to diagnose PIF on plain film. Bone scintigraphy is sensitive for the detection of PIF and shows the typical "H" pattern, which is produced when there are fractures of both sacral alae and a horizontal component involving the sacral body. We considered "H" sign on bone scintigraphy as diagnostic of PIF [10,21]. Fracture lines or sclerotic changes on CT scans are visible. CT should show that the remainder of the trabeculae are intact, rather than destroyed by a space-occupying lesion. Though sometimes the findings on CT scan are subtle and can be missed, it is helpful in confirming the diagnosis and excluding malignancy or osteomyelitis [21,22]. On MRI, presence of a fracture line on T1-weighted images and high signal intensity parallel to the sacroiliac joints on T2-weighted images are diagnostic of PIF [9,22-24]. Byun et al. [23] reported that a PIF appeared as low signal because of the bony sclerosis around fracture lines, whereas a pathologic fracture can show high signal intensity on diffusion-weighted images.
On PET/CT, PIF sites tend to have a lower standard uptake value (SUV) than malignant lesions. Many studies reported that the maximum SUV was lower than 5.6 [15,17-20]. Some authors, however, reported somewhat higher maximum SUV above 5.5 [5,16]. Shin et al. [20] reported maximum SUV of 0.6 to 5.5 (median, 2.9) in 15 patients of benign fractures. Oh et al. [18] reported 10 cases of sacral insufficiency fractures with maximum SUV of 2.4 to 7.2. They included two cases of intense uptake, 6.6 and 7.7. In our series, PIF sites showed a moderate increase of FDG uptakes with maximum SUV of 1.7 to 3.7 (Table 2). These results suggest that maximum SUV may help us to differentiate benign fractures from malignant condition, but it is not a perfect criterion by itself. The patterns of FDG uptakes can give us another important clue for differentiating insufficiency fractures from bone metastasis. Several studies described the patterns of FDG uptakes of benign fractures. Shin et al. [20] compared 19 cases of malignant and 15 cases of benign fractures. In benign fractures there was no uptake in the marrow in 14 of 15 cases, whereas malignant fractures demonstrated intramedullary FDG uptake [20]. Similarly, in our study, 12 of 13 patients showed no marrow uptake either. Some authors [7,18,25] reported that sacral fractures had a peculiar pattern to be differentiated easily from malignancy. In their reports, sacral fractures showed a characteristic pattern of diffuse and linear FDG uptake parallel to sacroiliac joint. In our study, all sacral fractures showed diffuse and linear uptake pattern parallel to sacroiliac joint.
The studies on duration of FDG uptake in benign fractures have been limited. Some authors reported that the FDG uptake decreased rapidly with time after fracture and that it was normalized within from 8 weeks to 3 months [26,27]. In our study, however, among 7 patients who underwent PET/CT at least twice after the diagnosis of PIF, 2 patients showed decreased but persistent uptakes after 12 and 27 months after radiotherapy (patient 10, 12). In one patient who had decreased but persistent uptake on the 12-month follow-up PET/CT, abnormal uptake on bone was normalized on the 16-month follow-up PET/CT (patient 10). Although we couldn't find exactly when the fractures developed, FDG uptake of radiation-induced insufficiency fractures might persist for more than 7 months. Interestingly one patient showed a new sacral uptake just near the original lesion on the 21-month follow-up PET/CT, which might be regarded as a new insufficiency fracture independent of previous PIF lesion (patient 3).
As mentioned above, bone scintigraphy, CT and MRI might be useful tools for diagnosing PIF. Several authors [9,10,21,22,24] evaluated the sensitivity and specificity of bone scintigraphy, CT and MRI. They reported that bone scintigraphy was highly sensitive and specific when typical "H" sign was present. They mentioned that when a typical "H" sign was absent, bone scintigraphy was much less specific. They also reported that CT was specific but might have limitations in sensitivity [21] and MRI was sensitive and specific tool for PIF. At our institution, only 22 patients underwent pelvic MRI and 9 patients underwent bone scintigraphy after the completion of radiotherapy. Before the adoption of PET/CT, most patients had been evaluated by pelvic CT alone at our institution unless they had a specific symptom like hip pain. That could account for quite low detection rate of PIF (3.8%) in the subgroup in which PET/CT was not done early.
The recent widespread use of PET/CT for the surveillance of malignancy makes it very important for clinicians to differentiate PIF from bony metastasis. Some authors [15,19,28] reported that PIF could be misdiagnosed as bony metastasis, which could lead to unnecessary tests for patients. Moreover, patients might undergo a biopsy or be treated by inappropriate chemotherapy or radiotherapy to their weakened bone. Donovan et al. [28] reported a case of unnecessary biopsy despite characteristic sclerotic change of bone. In our study, one asymptomatic patient's routine PET/CT showed a mild linear uptake in right sacral ala (patient 13). At that time we were not sure of PIF, so further evaluations by MRI, bone scintigraphy were done. Now we think MRI and bone scintigraphy might not be necessary for the diagnosis of PIF in that case.
There were some limitations in our study. Firstly, we didn't know when PIF developed because some patients were only diagnosed by regular follow-up imaging studies. Therefore we could not estimate the stage of each fracture. Secondly, because of its retrospective nature we couldn't get the same imaging studies at the same timing in all patients. Numbers of patients who underwent MRI or bone scintigraphy were too small, so we could not compare each imaging modality's sensitivity or specificity. Thirdly, although we included 235 patients in study pool, only 24 patients underwent BMD test and only 205 patients were available for BMI score, making interpretation of the statistical analysis somewhat difficult.
In conclusion, abnormally increased FDG uptakes on pelvic bone were common findings on follow-up PET/CT in patients who underwent pelvic radiotherapy with cervical cancer, which corresponded to PIFs. There was no case which finally turned out to be a bone metastasis. Mild diffuse FDG uptakes on pelvic bone after radiotherapy could be diagnosed as PIFs and should be followed by serial PET/CT. Adequate timely diagnosis and pain management for PIF can improve quality of life in patients with cervical cancer, in addition to reducing unnecessary medical expenses.
References2. Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA 2005;294:2587–2593, PMID: 16304072.
3. Henry AP, Lachmann E, Tunkel RS, Nagler W. Pelvic insufficiency fractures after irradiation: diagnosis, management, and rehabilitation. Arch Phys Med Rehabil 1996;77:414–416, PMID: 8607769.
4. Ogino I, Okamoto N, Ono Y, Kitamura T, Nakayama H. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol 2003;68:61–67, PMID: 12885453.
5. Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys 2008;70:1183–1188, PMID: 17919836.
6. Schmeler KM, Jhingran A, Iyer RB, et al. Pelvic fractures after radiotherapy for cervical cancer: implications for survivors. Cancer 2010;116:625–630, PMID: 20052724.
7. Igdem S, Alco G, Ercan T, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:818–823, PMID: 19879066.
8. Herman MP, Kopetz S, Bhosale PR, et al. Sacral insufficiency fractures after preoperative chemoradiation for rectal cancer: incidence, risk factors, and clinical course. Int J Radiat Oncol Biol Phys 2009;74:818–823, PMID: 19147305.
9. Blomlie V, Rofstad EK, Talle K, Sundfor K, Winderen M, Lien HH. Incidence of radiation-induced insufficiency fractures of the female pelvis: evaluation with MR imaging. AJR Am J Roentgenol 1996;167:1205–1210, PMID: 8911181.
10. Abe H, Nakamura M, Takahashi S, Maruoka S, Ogawa Y, Sakamoto K. Radiation-induced insufficiency fractures of the pelvis: evaluation with 99mTc-methylene diphosphonate scintigraphy. AJR Am J Roentgenol 1992;158:599–602, PMID: 1739002.
11. Konski A, Sowers M. Pelvic fractures following irradiation for endometrial carcinoma. Int J Radiat Oncol Biol Phys 1996;35:361–367, PMID: 8635945.
12. Tai P, Hammond A, Dyk JV, et al. Pelvic fractures following irradiation of endometrial and vaginal cancers-a case series and review of literature. Radiother Oncol 2000;56:23–28, PMID: 10869751.
13. Moreno A, Clemente J, Crespo C, et al. Pelvic insufficiency fractures in patients with pelvic irradiation. Int J Radiat Oncol Biol Phys 1999;44:61–66, PMID: 10219795.
14. Tsuchida T, Kosaka N, Sugimoto K, Itoh H. Sacral insufficiency fracture detected by FDG-PET/CT: report of 2 cases. Ann Nucl Med 2006;20:445–448, PMID: 16922475.
15. Fayad LM, Cohade C, Wahl RL, Fishman EK. Sacral fractures: a potential pitfall of FDG positron emission tomography. AJR Am J Roentgenol 2003;181:1239–1243, PMID: 14573412.
16. Ravenel JG, Gordon LL, Pope TL, Reed CE. FDG-PET uptake in occult acute pelvic fracture. Skeletal Radiol 2004;33:99–101, PMID: 14605771.
17. Halac M, Mut SS, Sonmezoglu K, Ylmaz MH, Ozer H, Uslu I. Avoidance of misinterpretation of an FDG positive sacral insufficiency fracture using PET/CT scans in a patient with endometrial cancer: a case report. Clin Nucl Med 2007;32:779–781, PMID: 17885357.
18. Oh D, Huh SJ, Lee SJ, Kwon JW. Variation in FDG uptake on PET in patients with radiation-induced pelvic insufficiency fractures: a review of 10 cases. Ann Nucl Med 2009;23:511–516, PMID: 19444551.
19. Salavati A, Shah V, Wang ZJ, Yeh BM, Costouros NG, Coakley FV. F-18 FDG PET/CT findings in postradiation pelvic insufficiency fracture. Clin Imaging 2011;35:139–142, PMID: 21377053.
20. Shin DS, Shon OJ, Byun SJ, Choi JH, Chun KA, Cho IH. Differentiation between malignant and benign pathologic fractures with F-18-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Skeletal Radiol 2008;37:415–421, PMID: 18309481.
21. Krestan C, Hojreh A. Imaging of insufficiency fractures. Eur J Radiol 2009;71:398–405, PMID: 19700255.
22. Cabarrus MC, Ambekar A, Lu Y, Link TM. MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol 2008;191:995–1001, PMID: 18806133.
23. Byun WM, Jang HW, Kim SW, Jang SH, Ahn SH, Ahn MW. Diffusion-weighted magnetic resonance imaging of sacral insufficiency fractures: comparison with metastases of the sacrum. Spine (Phila Pa 1976) 2007;32:E820–E824, PMID: 18091477.
24. Grangier C, Garcia J, Howarth NR, May M, Rossier P. Role of MRI in the diagnosis of insufficiency fractures of the sacrum and acetabular roof. Skeletal Radiol 1997;26:517–524, PMID: 9342810.
25. Huh SJ, Kim B, Kang MK, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol 2002;86:264–268, PMID: 12217746.
26. Zhuang H, Sam JW, Chacko TK, et al. Rapid normalization of osseous FDG uptake following traumatic or surgical fractures. Eur J Nucl Med Mol Imaging 2003;30:1096–1103, PMID: 12761597.
27. Shon IH, Fogelman I. F-18 FDG positron emission tomography and benign fractures. Clin Nucl Med 2003;28:171–175, PMID: 12592121.
28. Donovan A, Schweitzer ME, Rafii M, Lax A. Radiological features of superomedial iliac insufficiency fractures: a possible mimicker of metastatic disease. Skeletal Radiol 2009;38:43–49, PMID: 18682929.
Fig. 1The detection rate of pelvic insufficiency fracture (PIF) after pelvic radiotherapy in cervical cancer patients. Positron emission tomography/computed tomography (PET/CT) (+) means patients who underwent the first PET/CT in a year after radiotherapy. PET/CT (-) means patients who didn't undergo PET/CT in a year after radiotherapy. 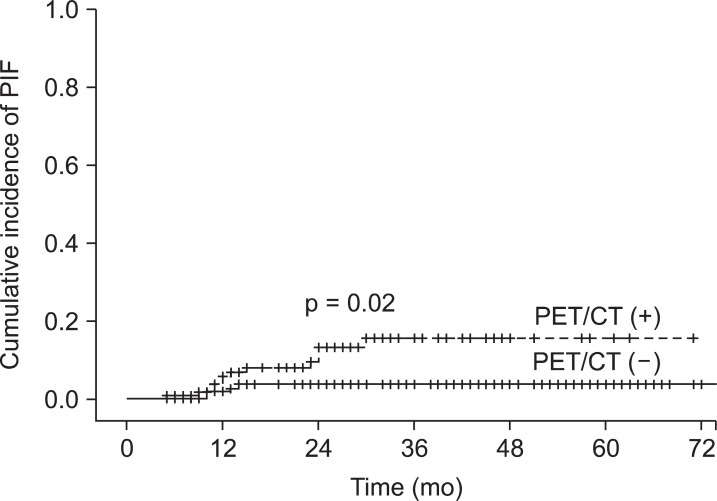
Fig. 2A 49-year-old woman (patient 6) who received definitive chemoradiotherapy for cervical cancer developed a hip pain at 11 months after radiotherapy. A diffuse mild linear flourodeoxyglucose (FDG) uptake (SUVmax, 3.3) parallel to sacroiliac joint in right sacral ala (arrow) was shown on maximum-intensity projection positron emission tomography (PET) scan (A), coronal fusion image (B) and axial fusion image (C). Follow-up PET/computed tomography at 20 months after radiotherapy showed normalized FDG uptake of sacroiliac joint (D-F). SUVmax, maximum standardized uptake value. 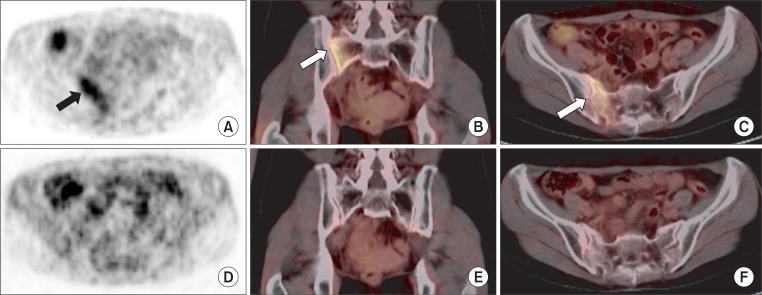
Fig. 3A 48-year-old woman (patient 3) who received definitive chemoradiotherapy for cervical cancer developed a hip pain at 12 months after radiotherapy. Serial positron emission tomography/computed tomography (PET/CT) scans at 12 months after radiotherapy (A-C) and 21 months after radiotherapy (D-F). A transverse linear mild flourodeoxyglucose (FDG) uptake (SUVmax, 1.7) in the sacrum (arrow) was shown on coronal fusion image at 12 months after radiotherapy (A). Follow-up PET/CT at 21 months after radiotherapy showed normalized transverse linear uptake (D) which was seen on previous PET/CT. A new vertical linear mild FDG uptake parallel to bilateral sacral alae (arrow) was shown on 21-month follow-up PET/CT (E,F), in which previous PET/CT scan showed normal finding (B,C). SUVmax, maximum standardized uptake value. 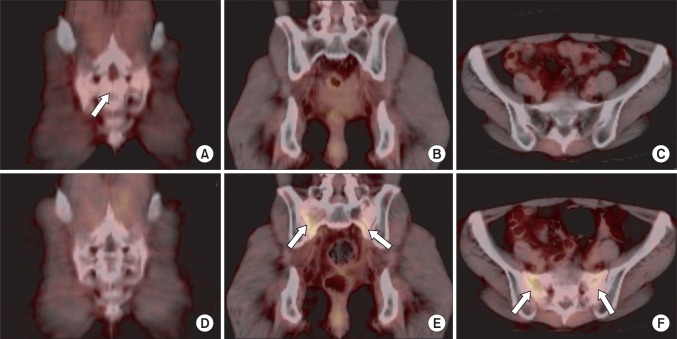
Table 1Characteristics of the patients 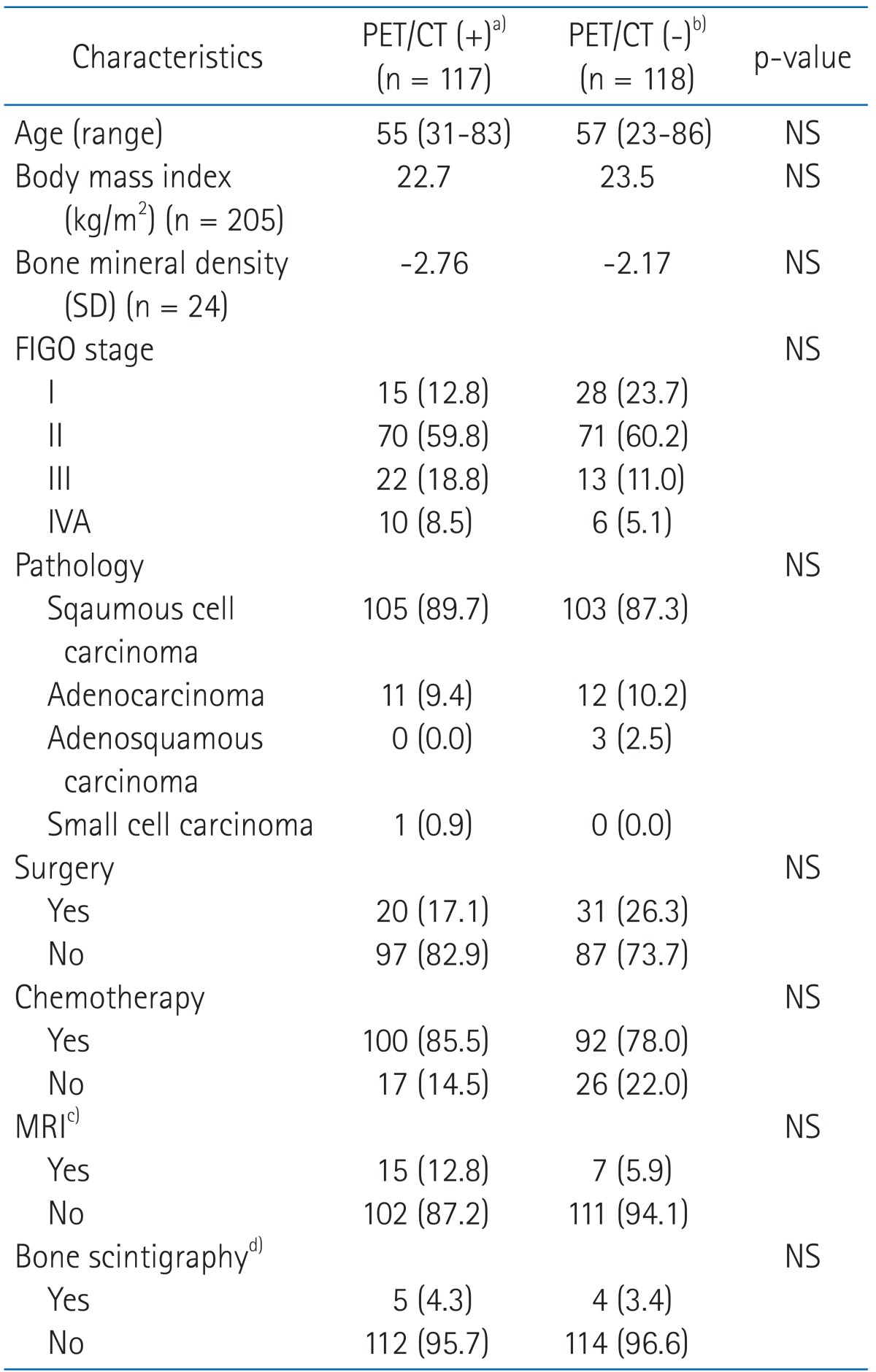 Values are presented as number (%) unless otherwise indicated. PET, positron emission tomography; CT, computed tomography; FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging; NS, not significant. a)Patients who underwent the first PET/CT within a year after completion of radiotherapy. b)Patients who didn't undergo the first PET/CT within a year after completion of radiotherapy. Among 118 patients, 36 underwent PET/CT after introduction of PET/CT at our institution. c)Patients who underwent MRI during the follow-up period. d)Patients who underwent bone scintigraphy during the follow-up period. |
|
||||||||||||||||||||||||||||||||||||

 |
 |