 |
 |
AbstractPurposeTo evaluate outcome and morbidity in patients with vulvar cancer treated with radiotherapy, concurrent chemoradiotherapy or postoperative radiotherapy.
Materials and MethodsThe records of 24 patients treated with radiotherapy for vulvar cancer between July 1993 and September 2009 were retrospectively reviewed. All patients received once daily 1.8-4 Gy fractions external beam radiotherapy to median 51.2 Gy (range, 19.8 to 81.6 Gy) on pelvis and inguinal nodes. Seven patients were treated with primary concurrent chemoradiotherapy, one patient was treated with primary radiotherapy alone, four patients received palliative radiotherapy, and twelve patients were treated with postoperative radiotherapy.
ResultsTwenty patients were eligible for response evaluation. Response rate was 55% (11/20). The 5-year disease free survival was 42.2% and 5-year overall survival was 46.2%, respectively. Fifty percent (12/24) experienced with acute skin complications of grade III or more during radiotherapy. Late complications were found in 8 patients. 50% (6/12) of patients treated with lymph node dissection experienced severe late complications. One patient died of sepsis from lymphedema. However, only 16.6% (2/12) of patients treated with primary radiotherapy developed late complications.
IntroductionVulvar cancer is a rare disease that represents only about 1% to 2% of all gynecological malignancy. Incidence of this malignancy is 0.2 cases per 100,000 women in the South Korea, 25.3% of patients with vulvar cancer are 70 years of age or older [1].
Surgery is the mainstay of treatment, with the majority of vulvar cancers being diagnosed at an early stage. Radical vulvectomy with dissection of bilateral inguinofemoral and pelvic lymph nodes improved overall survival but resulted in significant postoperative morbidities including wound disruption, chronic lymphedema, infection, urinary or sexual dysfunction [2,3]. Radiotherapy may require either as postoperative therapy for positive lymph node or primary treatment for unresectable disease [4-6]. Although postoperative radiotherapy is highly effective in preventing inguinal node recurrence [7], chronic severe complications such as lymphedema are common in patients who receive both surgery and radiotherapy [8]. Clearly restricting groin lymphadenectomy is the most obvious way of reducing groin and lower leg morbidities. In recent decades, there have been significant advances in multimodality therapy that reduce morbidity of inguinal node dissection. Numerous retrospective studies have shown therapeutic benefit with chemoradiotherapy in advanced vulvar cancer [9-12]. Landrum et al. [13] reported that 63 patients treated with primary chemoradiation and primary surgery had no differences in overall survival and progression free survival. The largest prospective phase II trial, the Gynecologic Oncology Group (GOG) 101 enrolled 71 patients with T3/T4 tumors and treated them with 2 cycles of 5-fluorouracil (5-FU) and cisplatin with radiation with a planned following surgical resection. Thirty-four of 71 of patients (48%) had complete response, and 70% of those had no residual tumor in histologic specimen [14]. The GOG 205 study is also investigating the outcome of patients treated with cisplatin based concurrent chemoradiotherapy and is currently under data review. At present, chemoradiation appears to be the treatment of choice for patients with locally advanced disease at diagnosis. The objective of this study was to review treatment pattern, outcome, pattern of recurrence, and treatment related morbidity in patients with vulvar cancer treated by radiotherapy, concurrent chemoradiotherapy or postoperative radiotherapy.
Materials and MethodsApproval was granted from the Institutional Review Board of our institution to proceed with this retrospective study. The records of patients treated with radiotherapy for vulvar cancer between July 1993 and September 2009 at Seoul St. Mary's Hospital and St. Vincent's Hospital were retrospectively reviewed. Clinical charts, operative reports, diagnostic imaging report, and pathology reports were assessed on all patients. Stages were assigned according to the 2009 the International Federation of Gynecology and Obstetrics (FIGO) staging system. Twenty seven patients with invasive vulvar cancer were identified from July 1993 to September 2009 at Seoul St. Mary's Hospital and St. Vincent's Hospital. One malignant melanoma and two squamous dysplasia were excluded in this study. Finally, 24 patients were included for analysis. The median age was 64.5 years (range, 39 to 89 years). The median follow-up was 59 months (range, 11 to 83 years). Seven patients were treated with primary concurrent chemoradiotherapy, 12 patients were treated with postoperative radiotherapy, 1 patient was treated with primary radiotherapy alone, and 4 patients received palliative radiotherapy. All patients received once daily 1.8-4 Gy fractions external beam radiotherapy median 51.2 Gy (range, 19.8 to 81.6 Gy) on pelvis and inguinal nodes. The evaluation for treatment response and recurrence was done based on either histology or any failure detected by physical examination and successive diagnostic imaging. Complete response (CR) was defined as no evidence of disease within the first follow up after radiotherapy. Partial response (PR) was defined as tumor regression of 50% or more but remaining disease within the same period. Disease free survival (DFS) and overall survival (OS) from the date of operation were estimated using the Kaplan-Meier method. Data were analyzed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Radiation morbidity was evaluated with Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) toxicity scoring scheme [15].
ResultsPatient's characteristics are presented in Table 1. Nineteen patients were diagnosed as squamous cell carcinoma, 2 patients were adenocarcinoma, 2 patients were extrammary Paget's disease, and 1 patient was adenocarcinoma with Paget's disease. Most patients (18/24) had lateral lesion located in labia major or labia minor. Twelve patients were stage I-II, 10 patients were stage III-IV, and 2 patients had recurrent disease, respectively. Almost patients had more than moderate performance status above Karnofsky performance status score 60.
Two patients had previous cervix cancer and 1 patient had anal cancer. One cervix cancer and 1 anal cancer previously treated with pelvic radiation. Seven patients were managed by primary chemoradiotherapy (Table 2). Twelve patients without adequate resection margin or with positive pelvic-inguinal lymph node received postoperative radiotherapy. Four patients with recurrent disease or distant metastasis received palliative radiotherapy. Chemotherapy was cisplatin based regimen. Radiation dose was median 51.2 Gy (range, 19.8 to 81.6 Gy). Seventeen patients received radiotherapy on both pelvis and bilateral inguinal region with photon and electron boost technique. Majority of patients (19/24) completed radiotherapy and 13 patients completed radiotherapy without treatment break. The main causes of unexpected withdrawal were poor tolerance due to acute skin reactions or decrease of general condition. We carried out the analysis according to the treatment intention.
Table 3 shows both treatment outcome and pattern of failure in 20 patients with vulvar cancer. Four patients were excluded for response evaluation because of 3 losses of follow up and 1 death at 2 weeks after radiotherapy. Eleven patients achieved CR, 4 patients achieved PR, and 5 patients developed stable disease (SD) or progression of disease (PD). Among the patients with CR, more than half of them (7/11) were treated with radical surgery and postoperative radiotherapy. Response rate was 55% (11/20) and was 75% (15/20) if included partial response. Eight of twenty patients showed recurrent disease after radiotherapy. Six of them developed locoregional recurrence. Two Patients treated with palliative radiotherapy developed both locoregional recurrence and distant metastasis. Among the patients with good treatment response (more than PR, 15 patients), 2 patients developed vulvar recurrence and 1 patient developed inguinal node recurrence. The 2-year OS was 72.6% and 5-year OS was 46.2%, respectively (Fig. 1A). The 2-year DFS was 63.2% and 5-year DFS was 42.2%, respectively (Fig. 1B). The 2-year OS of good response group (ŌēźPR) was 93.3% but 2-year OS of the others (SD + PD) was 31.3% (p = 0.0009) (Fig. 2). The median survival of patients with good response was 73 months but that was 13 months in patients with stable or progression of disease.
All of 24 patients showed acute skin complications. 12 patients (12 of 24) experienced moderate skin complications more than grade III during radiotherapy (Table 4). Skin complication caused treatment break longer than at least 1 week. Six patients with moderate skin complication had hold radiotherapy for the mean 2 weeks. Although the median radiotherapy dose was lower in the postoperative radiotherapy group (45 Gy vs. 56.1 Gy), there were more frequent treatment breaks in patients treated with postoperative radiotherapy (6/12) than in patients treated with chemoradiotherapy or radiotherapy (3/12). Two patients treated with concurrent chemoradiotherapy showed grade II leucopenia. Severe late complications were found in 8 patients. The percentage of 50 (6/12) of patients treated with both surgery and radiotherapy developed frequent severe late complication as wound disruption, lymphedema and cellulitis (Table 5). Among 6 patients treated with postoperative radiotherapy with late complications, 5 lymphedemas of ipsilateral leg developed at the mean 14 months after completion of radiotherapy and 2 wound disruptions developed. Finally, 1 patient died of infection from lymphedema. However, only 16.6% (2/12) of patients treated with primary radiotherapy developed late complications as 1 urethral stricture and 2 subcutaneous ulcerations.
Discussion and ConclusionTraditionally, radiotherapy has role in either postoperative adjuvant therapy or palliative therapy for recurrent disease. Kunos et al. [16] currently reported long term outcome and toxicities of postoperative inguinal-pelvic radiotherapy compared with pelvic dissection (GOG 37). From 45 to 50 Gy of radiotherapy significantly improved 6-year estimated survival in patients with clinically suspected or fixed ulcerated groin nodes (p = 0.004) and two or more positive groin nodes (p < 0.001). In our study, 9 patients treated with radical vulvectomy with bilateral inguinal node dissection followed postoperative radiotherapy. Only 2 of 9 patients had ipsilateral inguinal recurrence. Majority of patients with CR (7/11) were treated with radical surgery and postoperative radiotherapy.
Despite good local control of postoperative radiotherapy [17], we might feel worry about treatment complications because of well known morbidity of radical surgery. This study also showed that patients treated with postoperative radiotherapy experienced severe late complications more frequently than patients treated with radiotherapy (Table 5). Significant morbidity of inguinal node dissection addresses minimally invasive surgical technique as sentinel lymph biopsy. Van der Zee et al. [18] showed that treatment-related morbidity was minimal and survival was excellent in early stage patients performed with sentinel node biopsy. In 259 patients with unifocal vulvar disease and a negative sentinel node (median follow-up time, 35 months), six groin recurrences were diagnosed (2.3%; 95% confidence interval [CI], 0.6% to 5%), and 3-year survival rate was 97% (95% CI, 91% to 99%). Short-term morbidity was decreased in patients after sentinel node dissection only when compared with patients with a positive sentinel node who underwent inguinofemoral lymphadenectomy (wound breakdown in groin, 11.7% vs. 34.0%, respectively; p < 0.0001; and cellulitis, 4.5% vs. 21.3%, respectively; p < 0.0001). Long-term morbidity also was less frequently observed after removal of only the sentinel node compared with sentinel node removal and inguinofemoral lymphadenectomy (lymphedema of the legs, 1.9% vs. 25.2%, respectively; p < 0.0001).
Preoperative chemoradiotherapy can minimize the extent of surgery and give high rate of local control in patients with advanced vulvar cancer [10,14,19,20]. Moore et al. [14] reported the outcomes of 73 patients with clinical stage III-IV vulvar cancer (GOG 101). Split course of cisplatin/5-FU based concurrent radiotherapy resulted that 33/71 (46.5%) patients had no visible vulvar cancer at the time of planned surgery and 38/71 (53.5%) had gross residual cancer at the time of operation. Only 2/71 (2.8%) had residual unresectable disease. Toxicity was acceptable, with acute cutaneous reactions to chemoradiotherapy and surgical wound complications being the most common adverse effects. In our institutions, primary chemoradiotherapy has been performed in patients with unresectable disease or medically inoperable. Seven patients treated with cisplatin based concurrent chemoradiotherapy. Two patients (2/7) achieved CR and 3 patients (3/7) did PR, for overall response of 5 patients (5/7). Despite the overwhelming evidence in cervical and other squamous cancers of the therapeutic benefit, a recent large Surveillance Epidemiology, and End Results (SEER) review of treatment patterns of vulvar cancer revealed only 25% of women with advanced stage received concurrent chemotherapy with radiation [21]. As randomized prospective trials in vulvar cancer are not feasible, it will become increasingly important to consider using concurrent chemoradiotherapy strategies from the literature of other squamous cancers to help improve outcome.
Our data showed that all patients treated with conventional radiotherapy complained of acute skin reaction (Table 4). Thirteen patients (13/24) experienced grade III-IV acute skin reaction that resulted in more than 1 week treatment break. Beriwal et al. [19] reported a single institution's experience of patients with advanced vulvar cancer treated with preoperative chemotherapy and intensity-modulated radiotherapy (IMRT). It was found to help reducing dose to normal tissues such as the subcutaneous tissue, small bowel, bladder and rectum. Despite acute desquamations in the vulva and perineum were seen in all patients, no patient had grade III-IV of skin reaction in the groin region. In addition to reserving normal tissue, IMRT offers the opportunity to safely escalate the dose without significantly increasing the dose to critical organs. Thus, the approach using IMRT should be considered for prospective trial in a cooperative group setting with a sufficient number of patients and sufficiently long follow-up to determine its effect.
The current retrospective study has limitations in nature. Because of rarity of vulvar cancer, only 24 patients in two institutions for 16 years can be included for this study. Numbers of patients in each characteristic group are too small to attain statistical power. Numerous previous studies for vulvar cancer also had similar limitations [7,22-24]. Because of these limitations of this study all results should be interpreted with caution and serve mainly to help identify issue requiring prospective trials rather than to provide evidence based answers. National wide and multi-institutional prospective studies with sufficient patient numbers are needed to address some of the controversial issues pertinent to the management of vulvar cancer.
In summary, outcome of patients with vulvar cancer treated with radiotherapy showed relatively good local control and low recurrence. Severe late toxicities remained higher in patients treated with both node dissection and radiotherapy.
References1. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011;43:1ŌĆō11, PMID: 21509157.
2. Lin JY, DuBeshter B, Angel C, Dvoretsky PM. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol 1992;47:80ŌĆō86, PMID: 1427407.
3. Gould N, Kamelle S, Tillmanns T, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329ŌĆō332, PMID: 11531288.
4. Moore DH. Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: are two therapies better than one? Gynecol Oncol 2009;113:379ŌĆō383, PMID: 19232700.
5. Crosbie EJ, Slade RJ, Ahmed AS. The management of vulval cancer. Cancer Treat Rev 2009;35:533ŌĆō539, PMID: 19699036.
6. Wahlen SA, Slater JD, Wagner RJ, et al. Concurrent radiation therapy and chemotherapy in the treatment of primary squamous cell carcinoma of the vulva. Cancer 1995;75:2289ŌĆō2294, PMID: 7712439.
7. Katz A, Eifel PJ, Jhingran A, Levenback CF. The role of radiation therapy in preventing regional recurrences of invasive squamous cell carcinoma of the vulva. Int J Radiat Oncol Biol Phys 2003;57:409ŌĆō418, PMID: 12957252.
8. Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:683ŌĆō701, PMID: 12965139.
9. Landoni F, Maneo A, Zanetta G, et al. Concurrent preoperative chemotherapy with 5-fluorouracil and mitomycin C and radiotherapy (FUMIR) followed by limited surgery in locally advanced and recurrent vulvar carcinoma. Gynecol Oncol 1996;61:321ŌĆō327, PMID: 8641609.
10. Gerszten K, Selvaraj RN, Kelley J, Faul C. Preoperative chemoradiation for locally advanced carcinoma of the vulva. Gynecol Oncol 2005;99:640ŌĆō644, PMID: 16169579.
11. Lupi G, Raspagliesi F, Zucali R, et al. Combined preoperative chemoradiotherapy followed by radical surgery in loc ally advanced vulvar carcinoma: a pilot study. Cancer 1996;77:1472ŌĆō1478, PMID: 8608531.
12. Homesley HD, Bundy BN, Sedlis A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol 1991;164:997ŌĆō1003, PMID: 2014852.
13. Landrum LM, Skaggs V, Gould N, Walker JL, McMeekin DS. Comparison of outcome measures in patients with advanced squamous cell carcinoma of the vulva treated with surgery or primary chemoradiation. Gynecol Oncol 2008;108:584ŌĆō590, PMID: 18155755.
14. Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 1998;42:79ŌĆō85, PMID: 9747823.
15. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341ŌĆō1346, PMID: 7713792.
16. Kunos C, Simpkins F, Gibbons H, Tian C, Homesley H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet Gynecol 2009;114:537ŌĆō546, PMID: 19701032.
17. Jang WI, Wu HG, Park CI, et al. Management of regional lymph nodes in localized vulvar carcinoma. J Korean Soc Ther Radiol Oncol 2008;26:1ŌĆō9.
18. Van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 2008;26:884ŌĆō889, PMID: 18281661.
19. Beriwal S, Coon D, Heron DE, et al. Preoperative intensity-modulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol 2008;109:291ŌĆō295, PMID: 18455637.
20. Montana GS, Thomas GM, Moore DH, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2000;48:1007ŌĆō1013, PMID: 11072157.
21. Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 2008;108:577ŌĆō583, PMID: 18155274.
22. Talaat A, Brinkmann D, Nagar Y, Hogston P, Khoury G, Woolas R. Experience in the management of patients older than 80 years with vulval cancer. Int J Gynecol Cancer 2009;19:752ŌĆō755, PMID: 19509583.
23. Hou JL, Wu LY, Zhang HT, Lv NN, Huang Y, Yu GZ. Clinicopathologic characteristics of 12 patients with vulvar sweat gland carcinoma. Int J Gynecol Cancer 2010;20:874ŌĆō878, PMID: 20606537.
24. Tans L, Ansink AC, van Rooij PH, Kleijnen C, Mens JW. The role of chemo-radiotherapy in the management of locally advanced carcinoma of the vulva: single institutional experience and review of literature. Am J Clin Oncol 2011;34:22ŌĆō26, PMID: 20087157.
Fig.┬Ā1Kaplan-Meier survival analysis; overall survival (A) and disease free survival (B) of patients with vulvar cancer treated with radiotherapy (n = 20). 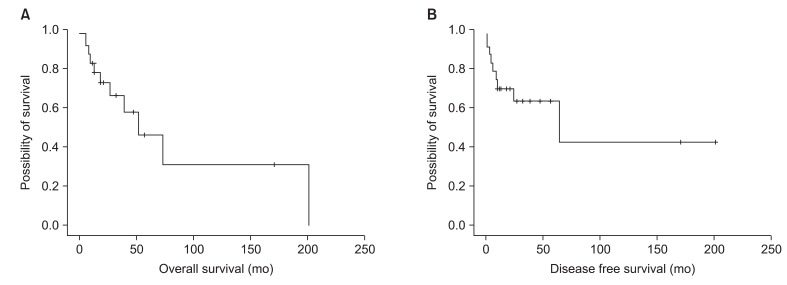
Fig.┬Ā2Overall survival of patients with vulvar cancer according to treatment response. PR, partial reponse; SD, stable disease; PD, progressed disease. 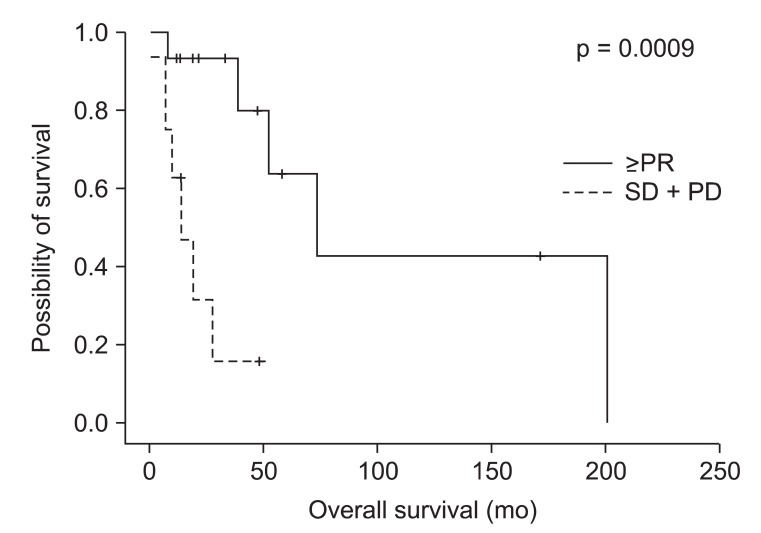
|
|
||||||||||||||||||||||||||||||||||||

 |
 |