Regional nodal irradiation in pT1-2N1 breast cancer patients treated with breast-conserving surgery and whole breast irradiation
Article information
Abstract
Purpose
To evaluate the necessity of regional nodal irradiation (RNI) for pT1-2N1 breast cancer patients treated with breast-conserving surgery and radiotherapy, we compared clinical outcomes of patients treated with and without RNI.
Materials and Methods
We retrospectively analyzed the data of 214 pT1-2N1 breast cancer patients treated with breast-conserving surgery and whole breast irradiation from 2007–2016. There were 142 (66.4%), 51 (23.85%), and 21 (9.8%) patients with one, two, and three positive lymph nodes, respectively. Thirty-six patients (16.8%) underwent RNI. Adjuvant chemotherapy, endocrine therapy, and anti-HER2 therapy were given to 91.6%, 79.0%, and 15.0% patients, respectively. The most common chemotherapy regimen was anthracycline + cyclophosphamide, followed by taxane (76.5%). The median follow-up was 64 months (range, 6 to 147 months). Patients were propensity matched 1:2 into RNI and no-RNI groups.
Results
Two patients experienced locoregional recurrences simultaneously with distant metastases, ten patients developed distant metastases, and one patient died. Before matching, the 5-year actuarial locoregional control (LRC), distant metastasis-free survival (DMFS), and overall survival (OS) rates in the RNI and no-RNI groups were 100.0% and 99.4% (p = 0.629), 94.1% and 96.0% (p = 0.676), and 100.0% and 99.4% (p = 0.658), respectively. After matching, the 5-year LRC, DMFS, and OS were 98.3% and 100.0% (p = 0.455), 96.6% and 93.9% (p = 0.557), and 100.0% and 100.0% (p > 0.999) in the RNI and no-RNI groups, respectively. No clinicopathologic or treatment-related factors were significantly associated with LRC, DMFS, or OS.
Conclusion
Adding RNI did not show superior LRC, DMFS, or OS in pT1-2N1 breast cancer patients.
Introduction
Breast cancer is the most common cancer among women both worldwide and in Korea [1,2]. It is also the most common cause of cancer-related deaths worldwide women and the 6th most common cause of cancer-related deaths among Korean women. In 2016, the age-standardized ratio for breast cancer incidence and cancer-related deaths was 54.9 and 9.6 per 100,000, respectively, in Korea [2].
In contrast to the increasing incidence in Korea, globally, the overall locoregional recurrence (LRR) rate of breast cancer has decreased substantially over the recent decades [3-5]. Meanwhile, breast-conserving surgery with breast radiotherapy has been the standard treatment for women with early-stage breast cancer. Compared with breast-conserving surgery alone, adjuvant radiotherapy following breast-conserving surgery has been proven to enhance locoregional control (LRC) and prolong survival in multiple randomized controlled trials [6-10].
Although it is known that adjuvant radiotherapy for breast cancer plays an important role in patients who have undergone breast-conserving surgery, the necessity of elective regional nodal irradiation (RNI) for patients with one to three positive nodes remains controversial. The National Comprehensive Cancer Network (NCCN) guidelines strongly recommend RNI even for pN1 patients [11]. Two randomized clinical trials published in 2015 showed the effectiveness of RNI in patients with early-stage breast cancer who underwent breast-conserving surgery [12,13]. However, these studies included patients who were treated between 1996 and 2007. Since then, diagnostic and therapeutic paradigms for breast cancer have evolved significantly. Consequently, the role of elective RNI is still questioned because it may increase radiation-associated morbidity. With this background, we analyzed the clinical outcomes of patients with pT1-2N1 breast cancer who underwent breast-conserving surgery and adjuvant breast irradiation with or without elective RNI.
Materials and Methods
1. Patients
We retrospectively reviewed the medical records of 436 consecutive patients who had pathologically proven breast cancer with a ≤5 cm tumor and one to three positive axillary lymph nodes at our institution between 2007 and 2016. The Institutional Review Board of Kyungpook National University Chilgok Hospital approved this study and provided a waiver of consent (No. 2018-03-018). Of 436 patients, 231 and 205 underwent breast-conserving surgery and mastectomy, respectively. From the 231 patients treated with breast-conserving surgery, we excluded patients who refused adjuvant breast radiotherapy (n = 8) or were followed-up for a short duration (<6 months) after surgery (n = 9). Finally, 214 patients were included in the analysis (Fig. 1).
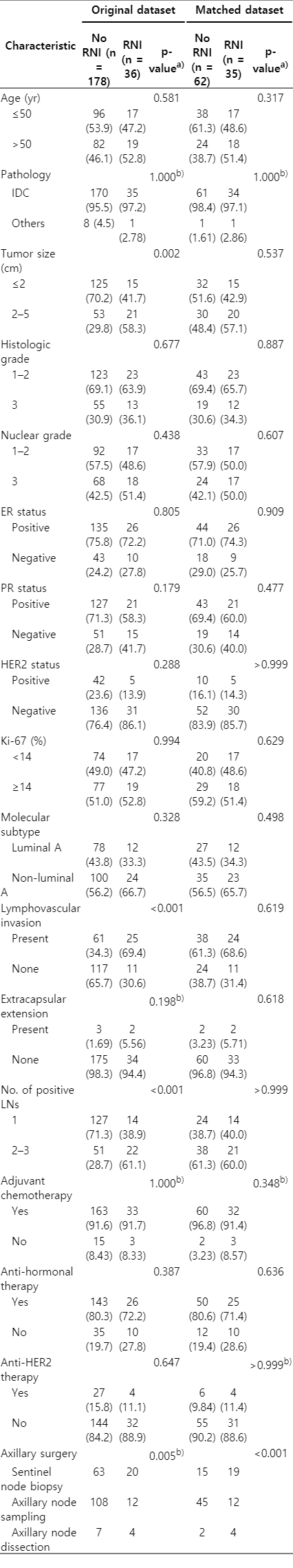
Patient, tumor, and treatment characteristics for the 214 patients are listed in Table 1. The median age was 49 years (range, 29 to 78 years). The median follow-up period was 64 months (range, 6 to 147 months). Tumor size was ≤2 cm in 140 patients (65.4%) and 2–5 cm in 74 patients (34.6%). Lymphovascular invasion and extracapsular extension were observed in 86 (40.6%) and 5 patients (2.3%), respectively. There were 142 (66.4%), 51 (23.85%), and 21 (9.8%) patients with one, two, and three positive lymph nodes, respectively. Molecular subtypes were luminal A, luminal B, human epidermal growth factor receptor 2 (HER2), and basal type in 90 (42.1%), 74 (34.6%), 11 (5.1%), and 39 (18.2%) patients, respectively. The expression of estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 was scored based on the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines [14,15]. Molecular subtypes were categorized as follows: (1) luminal A: either ER or PR positive, HER2 negative, and Ki-67 <14%; (2) luminal B: either ER or PR positive, and either HER2 positive or HER2 negative with Ki-67 ≥14%; (3) HER2: ER and PR negative and HER2 positive; and (4) basal: ER, PR, and HER2 negative [16].
2. Treatments
All patients underwent breast conserving surgery and sentinel lymph node biopsy. Subsequent axillary lymph node dissection (ALND) was performed when the patient agreed to the procedure. The extent of ALND included level I and II axillary lymph nodes. The median number of removed axillary lymph nodes was 9 (range, 1 to 35).
The median dose of adjuvant radiotherapy for the ipsilateral whole breast was 50.4 Gy (range, 50 to 50.4 Gy). The median tumor bed boost dose was 10 Gy (range, 10 to 16 Gy). In patients who received ALND, the axillary region was not included in the clinical target volume (CTV), whereas the CTV included level I and II axilla in patients who did not receive ALND. The implementation of RNI was discussed by a multidisciplinary breast cancer team for every patient while considering risk factors for LRR, such as lymphovascular invasion, three involved axillary lymph nodes, or level II or III axillary lymph node involvement; only then was it finally decided at the discretion of the treating radiation oncologist [17-19]. The CTV for RNI generally included supraclavicular fossa, but the internal mammary nodal region was also considered to be included in patients with medial breast cancer. Elective RNI to the supraclavicular fossa or internal mammary region was performed for 36 patients (16.8%): supraclavicular irradiation for 34 patients and supraclavicular combined with internal mammary irradiation for 2 patients. After breast-conserving surgery and adjuvant radiotherapy, adjuvant chemotherapy, anti-hormonal therapy, and anti-HER2 therapy were given to 196 (91.6%), 169 (79.0%), and 31 (15.0%) patients, respectively. The most common chemotherapy regimen was anthracycline + cyclophosphamide, followed by taxane (n = 150, 76.5%).
We defined two treatment groups: the no-RNI and RNI groups. The former included patients who received whole-breast irradiation only, and the latter included patients who received whole-breast irradiation plus elective RNI to their supraclavicular fossa or internal mammary region. In the original dataset, there were significant differences in tumor size, lymphovascular invasion, and the number of positive lymph nodes between the no-RNI and RNI groups (Table 1). Patients in the RNI group were more likely to have lymphovascular invasion, two to three positive lymph nodes, and tumors larger than 2 cm in diameter.
3. Propensity score matching
To balance the imbalanced covariates between the RNI and no-RNI groups, we matched the treatment groups using propensity score analysis. The propensity score was estimated as the predicted probability of a patient being in the RNI group from a logistic regression model. The propensity score model included tumor size, lymphovascular invasion, and the number of positive lymph nodes. Patients were propensity matched 1:2 into RNI and no-RNI groups using the nearest-neighbor method with a caliper width of 0.1 (maximum allowable difference in propensity scores). Thirty-five patients from the RNI group were matched with 62 patients from the no-RNI group. The balance in the covariates in the matched data was examined using standardized differences. The distribution of baseline variables was balanced in the matched dataset (Table 1). The largest standardized difference was 0.087 in the matched dataset.
4. Statistical analysis
Potential categorical confounding variables between treatment groups were compared using the χ2 test or Fisher exact test. The primary endpoint of this study was to compare the LRC rates in the RNI and no-RNI groups. The secondary endpoints were to compare the groups in terms of distant metastasis risk and overall survival (OS). LRR was defined as an ipsilateral chest wall recurrence or recurrence involving at least one ipsilateral axillary lymph node, internal mammary lymph node, or supraclavicular lymph node. Distant metastasis was defined as metastasis to any distant site. Disease-free survival (DFS) was defined as the interval between surgery and any disease recurrence or death from any cause. All endpoints were calculated from the date of breast-conserving surgery and were estimated using the Kaplan-Meier method. Statistical differences were compared using the log-rank test in a univariate analysis. Multivariate analysis using Cox proportional hazard modeling was performed to identify independent predictors among the prognostic factors. p-values <0.05 were considered statistically significant. All statistical analyses were performed using R statistical software version 3.5.1 (The R Project, Vienna, Austria; http://www.R-project.org). Matching was processed using the MatchIt package [20,21].
Results
1. Locoregional recurrence
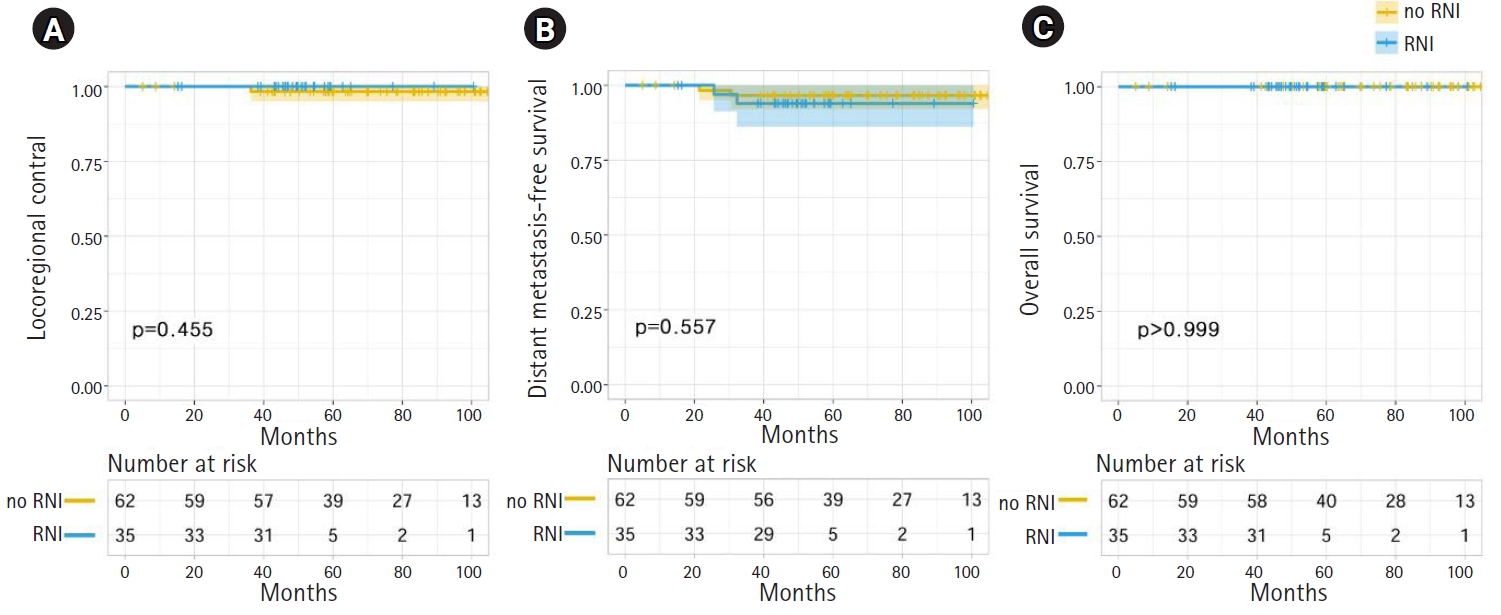
No patient developed isolated LRR during follow-up. Two patients (0.1%) experienced LRR simultaneously with distant metastasis (Table 2). One patient initially had a 21-mm invasive ductal carcinoma with metastasis to one axillary lymph node. She developed metastases in level III axillary lymph nodes and supraclavicular lymph nodes at 36 months after surgery. The other patient who experienced LRR initially had a 20-mm invasive ductal carcinoma with metastasis to one axillary lymph node; LRR involved the supraclavicular lymph nodes at 71 postoperative months. Both patients were diagnosed with concurrent distant metastasis and LRR. The 5-year actuarial LRC rates were 100.0% and 99.4% in the RNI and no-RNI groups, respectively (p = 0.629) (Fig. 2A). No clinicopathologic or treatment factors were found to impact LRC in either univariate or multivariate analyses.
2. Distant metastasis-free survival
Eight patients developed isolated distant metastasis, and 2 patients had concurrent distant metastasis and LRR. The median time to distant metastasis was 25.8 months (range, 7 to 71 months). The sites of distant metastasis were the liver in 3 patients, bone in 2 patients, lung in 2 patients, and multiple sites in 3 patients. The 5-year actuarial distant metastasis-free survival (DMFS) rates were 94.1% and 96.0% in the RNI and no-RNI groups, respectively (p = 0.676) (Fig. 2B). In univariate and multivariate analyses, there were no clinicopathologic or treatment factors significantly associated with DMFS.
3. Overall survival
One patient (0.5%) died during the follow-up period. The cause of death was chemotherapy-related pneumonia during the last planned adjuvant chemotherapy cycle of taxane and cyclophosphamide. The 5-year actuarial OS rates were 100.0% and 99.4% in the RNI and no-RNI groups, respectively (p = 0.658) (Fig. 2C). There were no clinicopathologic or treatment factors associated with OS in univariate and multivariate analyses.
4. LRC, DMFS, and OS after matching
In the propensity score-matched dataset, there were no significant differences between the RNI and no-RNI groups in terms of LRC, DMFS, and OS (Fig. 3). The LRC, DMFS, and OS rates of no-RNI and RNI groups at 5 years were 98.3% and 100.0% (p = 0.455), 96.6% and 93.9% (p = 0.557), and 100.0% and 100.0% (p > 0.999), respectively. No clinicopathologic or treatment factors were significantly associated with LRC, DMFS, or OS in univariate and multivariate analyses.
5. Treatment-related toxicity
Lymphedema developed in 20 patients (9.3%): 15 were stage 0, and 5 were stage 1, according to the International Society of Lymphology staging system [22]. There were no significant differences in the incidence of lymphedema between the no-RNI and RNI groups (Table 2).
No patient developed symptomatic radiation pneumonitis. However, radiologic lung density changes on computed tomography at 4–24 weeks after radiotherapy were observed in 94 patients (43.9%). These radiologic abnormalities included ground-glass opacities, patchy consolidation, and lung fibrosis in the irradiated lung fields. The incidence of radiologic lung density changes was significantly higher in the RNI group than in the no-RNI group (63.9% vs. 39.9%, respectively; p = 0.014) (Table 2). After propensity score matching, the RNI group showed higher incidence of radiation pneumonitis than the no-RNI group (62.9% vs. 43.5%, respectively); however, the difference was not statistically significant (p = 0.106) (Table 2).
Discussion and Conclusion
The results of this study suggest that patients who underwent elective RNI do not exhibit improved LRC, DMFS, or OS compared with patients who underwent whole-breast irradiation without elective RNI. With a median follow-up period of 64 months, only 2 patients out of 214 included patients had LRR.
Our results seem to contradict the results of two randomized controlled trials, which suggested that adding RNI to whole-breast irradiation improves DFS in patients with early-stage breast cancer. The National Cancer Institute of Canada Clinical Trial group (MA.20 NCI-C) [23] showed that elective RNI was associated with superior DFS but not with OS. These results were consistent with the results of the European Organization for Research and Treatment of Cancer (EORTC) 22922-10925 trial [13], which showed that elective RNI was associated with superior DFS and DMFS. These results suggested that adding elective RNI to whole-breast irradiation is necessary to reduce LRR and increase DFS in breast-conserved patients with early breast cancer.
Conversely, several retrospective series have favored forgoing RNI for pN1 breast cancer patients. The Korean Radiation Oncology Group (KROG 14-18) conducted a multi-institution case-control study comparing whole-breast irradiation with and without supraclavicular irradiation in patients who underwent breast-conserving surgery, adjuvant breast irradiation, and anthracycline or taxane chemotherapy [24]. Supraclavicular recurrences developed in 3 patients. All of these patients had simultaneous distant metastases, just like the 2 patients who experienced LRR in our study. The 5-year DFS rates were 94.4% and 92.6% in patients with and without supraclavicular irradiation, respectively (p = 0.50). The authors concluded that there was no benefit associated with the addition of supraclavicular irradiation for patients treated with a modern systemic therapy regimen. Similarly, Biancosino et al. [25] compared clinical outcomes among pN1 breast cancer patients who underwent breast-conserving surgery and breast irradiation with or without periclavicular irradiation. The authors reported no statistical differences in terms of LRR-free survival, DFS, or OS. In a retrospective study, Livi et al. [26] reported outcomes in 5717 patients who underwent breast-conserving surgery and breast irradiation without RNI. In their cohort, 24 of 1,107 patients with pN1 disease (2.1%) had supraclavicular nodal failure, while 133 patients (12.0%) had distant metastases. The authors concluded that it is reasonable to focus on improving the risk of distant metastasis for these patients.
Some researchers doubt that the risk of LRR in patients treated in the modern treatment era is sufficiently high to justify the use of elective RNI. Compared with findings from previous decades, recent studies have reported lower levels of LRR risk [3,9,27]. In our study, only 2 patients had LRR, and the 5-year LRC rate of the no-RNI group was 99.4%. Moreover, no patient had isolated LRR during the follow-up period. Our findings are in line with those of a previously reported analysis of the EROTC/BIG 03-4 MINDACT trial [28]. In this trial, a 5-year LRR rate of 2.1% was observed among 5,470 patients who underwent breast cancer surgery and adjuvant radiotherapy. Similar findings were observed in a retrospective analysis conducted by Hirata et al. [27]. In their study, the 5- and 10-year regional recurrence-free survival rates were 97.4% and 93.7%, respectively, among patients who were treated with breast-conserving treatment without RNI. Reddy and Kiel [29] also reported a 10-year supraclavicular nodal failure rate of 2.08% among N1 patients in their cohort, suggesting that adding supraclavicular irradiation for N1 patients was unnecessary. These findings reveal that over the last 2 decades, the overall LRR rates of early breast cancer have decreased substantially, owing to the contributions of effective systemic therapy and radiotherapy [30]. Thus, the necessity of RNI for patients, despite an LRR risk lower than 3%, remains controversial.
RNI may increase radiotherapy-related morbidity. In our study, radiologic lung changes on computed tomography after radiotherapy were more commonly found in the RNI group than in the no-RNI group (63.9% vs. 39.9%, respectively). Although neither group presented with symptomatic radiation pneumonitis, radiologic lung changes could be considered an objective end point for evaluating radiation-induced lung toxicity, thus implying their clinical significance [31]. In terms of lymphedema, no difference was found between the RNI and no-RNI groups in our study. Conversely, some investigators have reported increased radiotherapy-related morbidity among patients having undergone RNI. Chua et al. [32] noted that adding RNI to breast irradiation was associated with an increased incidence of lymphedema and symptomatic radiation pneumonitis. In the aforementioned KROG 14-18 study [24], adding RNI was associated with an increased risk of lymphedema and radiation pneumonitis. The incidence of lymphedema was 16.6% among patients who underwent RNI and 10.7% among patients who did not undergo RNI. Given these findings, in the modern treatment era, the benefits of adding RNI might not be enough to justify the associated increased risk of radiation-induced complications.
Our study had several limitations. First, there may have been a concealed selection bias brought about by the retrospective design, even though we performed a propensity score-matched analysis to adjust for the differences between the groups. The effect of a potential selection bias may have been minimized by the fact that our study included consecutive patients who were homogeneously treated according to a consistent protocol at a single institution. Second, the relatively small sample size from a single institution means that caution should be taken when interpreting and generalizing the results. Third, with a median duration of 60 months, the follow-up period was relatively short and may not have been long enough to detect some recurrences or metastases. Nevertheless, our study provides meaningful information about clinical outcomes among patients with pT1-2N1 breast cancer who underwent breast-conserving surgery and radiotherapy followed by modern systemic treatment.
In conclusion, our results suggest that adding RNI may not be associated with superior LRC, DMFS, or OS. However, considering the retrospective design and small number of patients included in the study, our results must be interpreted with caution, and further study is warranted to define the impact of RNI on pT1-2N1 breast-conserved patients.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.