Feasibility and safety of neck level IB-sparing radiotherapy in nasopharyngeal cancer: a long-term single institution analysis
Article information
Abstract
Purpose
Nasopharyngeal cancer (NPC) has a higher prevalence of regional nodal metastasis than other head and neck cancers; however, level IB lymph node involvement is rare. We evaluated the safety and feasibility of level IB-sparing radiotherapy (RT) for NPC patients.
Materials and Methods
We retrospectively reviewed 236 patients with NPC who underwent definitive intensity-modulated RT with or without chemotherapy between 2004 and 2018. Of them, 212 received IB-sparing RT, and 24 received non-IB-sparing RT. We conducted a propensity score matching analysis to compare treatment outcomes according to IB-sparing status. In addition, dosimetric analysis of the salivary glands was performed to identify the relationship between xerostomia and the IB-sparing RT.
Results
The median follow-up duration was 78 months (range, 7 to 194 months). Local, regional, and distant recurrences were observed in 11.9%, 6.8%, and 16.1% of patients, respectively. Of the 16 patients with regional recurrence, 14 underwent IB-sparing RT. The most common site categorization of regional recurrence was level II (75%), followed by retropharyngeal lymph nodes (43.8%); however, there was no recurrence at level IB. In the matched cohorts, IB-sparing RT was not significantly related to treatment outcomes. However, IB-sparing RT patients received a significantly lower mean ipsilateral and contralateral submandibular glands doses (all, p < 0.001) and had a lower incidence of chronic xerostomia compared with non-IB-sparing RT patients (p = 0.006).
Conclusion
Our results demonstrated that IB-sparing RT is sufficiently safe and feasible for treating NPC. To reduce the occurrence of xerostomia, IB-sparing RT should be considered without compromising target coverage.
Introduction
Radiotherapy (RT), with or without chemotherapy, is the mainstay treatment modality for nasopharyngeal cancer (NPC), owing to NPC’s anatomical and histological characteristics [1–4]. Cervical lymphatic metastasis is common in NPC patients, and it has been reported that lymphadenopathy is present at diagnosis in 85% of patients [5]. In particular, in the neck, level II and retropharyngeal lymph nodes (RLNs) are the most commonly involved sites for regional metastasis [5,6].
In contrast, lymph node metastasis at level IB is rare and has a 2%–4% reported incidence [6,7]. Therefore, the inclusion of level IB in elective neck irradiation for NPC patients is still controversial. Some studies have routinely included level IB in the RT field [8,9], while others have recommended selectively sparing level IB in elective RT (IB-sparing RT) of NPC [10,11]. Additionally, recent studies have found IB-sparing RT using the intensity-modulated RT (IMRT) technique to be safe and feasible for selected NPC patients [12,13].
Xerostomia is the most common late toxicity associated with RT treatment for NPC [13]. Furthermore, xerostomia may cause impaired taste, swallowing and chewing difficulties, or increased dental caries, which are determinants of quality of life [14,15]. In saliva production, stimulated saliva is mainly produced by the parotid glands. In contrast, the submandibular glands (SMGs) are associated with unstimulated saliva and mucus, which can influence the degree of dry mouth sensation [16,17]. Of these salivary glands, the SMGs are located in neck node level IB, so we considered IB-sparing RT to reduce the SMG dose and subsequently decrease the incidence of xerostomia.
This study evaluated the safety, feasibility, and toxicity-reduction effects of IB-sparing RT in the treatment of NPC. Mainly, we tried to assess detailed treatment outcomes, the effect of SMG dose reduction, and the incidence of xerostomia after IB-sparing RT.
Materials and Methods
1. Patient selection
We retrospectively reviewed 251 patients with NPC who underwent curative-intent RT with or without chemotherapy between January 2004 and December 2018 at a single institution. All patients had biopsy-proven NPC before initiating treatment, and all underwent computed tomography (CT) and/or magnetic resonance imaging (MRI) of the head and neck at diagnosis. Additionally, chest X-ray, chest CT, and/or abdominal CT were performed for suspicious lesions, and positron emission tomography-computed tomography (PET-CT) was performed for almost all cases.
The exclusion criteria were as follows: patients with distant metastases at diagnosis (n = 5), with a previous diagnosis of primary cancer (n = 2), or lost to follow-up after treatment (n = 8). Ultimately, 236 patients were enrolled in this study. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2012-192-1187), which waived the requirement for informed consent due to the retrospective study design.
2. Radiotherapy and chemotherapy
All patients underwent CT simulation with a 3-mm slice thickness using intravenous contrast and were treated with IMRT. Patients were immobilized in the supine position during the simulation and treatment, with the neck extended in a thermoplastic head-and-shoulder mask.
The gross tumor volume (GTV) included the primary tumor and positive regional lymph nodes, which were identified by CT, MRI, physical exam, or nasopharyngoscopy. The high-risk clinical target volume (CTV) was expanded in all directions by 5 mm relative to the GTV and modified according to anatomical structure. The intermediate-risk CTV included the entire nasopharyngeal mucosa and adjacent normal structures, such as the parapharyngeal space, retropharyngeal space, pterygopalatine fossa, sphenoid sinus, pterygoid fossa, cavernous sinus, and skull base, according to the extents of the primary disease and included cervical nodal stations that involved positive lymph node with or without 1 subsequent uninvolved lymph node level. The low-risk CTV included bilateral cervical nodes that were not covered by the high- and intermediate-risk CTVs. Recently, there has been a tendency to exclude level IV of the uninvolved side. However, levels IA and IB were not routinely covered by the CTVs unless there was disease involvement of the corresponding lymph node level. Elective level IB irradiation was determined by the radiation oncologist, mainly based on these factors as follows: N stage, maximal diameter of level IIA, extracapsular spread of level IIA, and multiple neck level involvement.
Finally, in our study, 212 patients (89.8%) underwent level IB-sparing RT, and 24 patients (10.2%) underwent non-IB-sparing RT. The planning target volumes (PTVs) were expanded by 3-mm auto-expansion to the CTVs. The total doses of high-, intermediate-, and low-risk PTVs were 67.5 Gy, 54–60 Gy, and 48 Gy in 30 daily fractions, respectively. RT was delivered 5 days per week. The dose constraints for adjacent organs-at-risk followed the Radiation Therapy Oncology Group guidelines: brain stem maximum dose (Dmax) ≤54 Gy; optic nerve and chiasm Dmax ≤54 Gy; spinal cord Dmax ≤45 Gy; parotid glands V30 ≤50% (where Vx is the percentage of the structure volume exceeding x Gy). However, no dose constraints were applied to the other organs, including SMG.
In total, 214 patients (90.7%) received chemotherapy, and the remainder were treated with RT alone. Of the patients who received chemotherapy, 94.9% (n = 203) received concurrent chemoradiotherapy with or without neoadjuvant/adjuvant chemotherapy and 5.1% (n = 11) received sequential chemoradiotherapy. The use of neoadjuvant and/or adjuvant chemotherapy was determined by the multidisciplinary team, and all regimens mainly used cisplatin-based chemotherapy.
3. Assessment and follow-up
Patients were evaluated weekly during RT and visited the outpatient clinic 2 weeks after completion of RT. Then, patients were examined at 1- or 2-month intervals for the first year, every 3 months in the second year, every 6 months for the next 3 years, and annually thereafter. At every visit, the patients underwent physical examination, flexible fiberoptic nasopharyngoscopy, and imaging studies (ultrasonography, CT, MRI, or PET-CT).
Treatment-related toxicities were retrospectively graded using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 according to the physician’s assessment. Late toxicity was defined as any toxicity occurring at least 6 months after the completion of RT. In terms of xerostomia, we evaluated the incidence and grade according to the extent of level IB sparing. In addition, we compared the SMGs and parotid glands doses between level IB-sparing RT and non-IB-sparing RT in patients (n = 155) whose dosimetric data were available. Fig. 1 shows the difference in dosimetric plans according to the sparing of neck level IB.
4. Statistical analysis
Regional recurrence-free survival (RRFS) and locoregional recurrence-free survival (LRRFS) were defined from the start of treatment until the date of regional failure and locoregional failure (LRF), respectively. Progression-free survival (PFS) was calculated as the time from treatment initiation until LRF, distant metastasis, or death, whichever occurred first. Overall survival (OS) was defined as the time from treatment initiation to the date of death. To control for differences in characteristics between the two groups according to level IB-sparing status, we performed the propensity score matching analysis. The variables used in PSM were as follows: age, N stage, the American Joint Committee on Cancer (AJCC) stage, bilateral neck node involvement, and status of level IIA lymph nodes (size and extracapsular spread). Using propensity scores, the IB-sparing RT group and non-IB-sparing RT group were matched with a 3:1 nearest-neighbor matching algorithm with a caliper width of 0.5 standard deviations. The survival rates were analyzed using the Kaplan-Meier method and were compared using the log-rank test. The dosimetric parameters included the mean dose to the ipsilateral and contralateral SMGs, mean dose to the parotid glands, V20 of parotid glands and V30 of parotid glands. Student t-test and the chi-square test were performed to compare variables according to IB-sparing status. We defined statistical significance as a p-value <0.05. All statistical analyses were conducted using Stata 17.0 (StataCorp, College Station, TX, USA).
Results
1. Patient characteristics
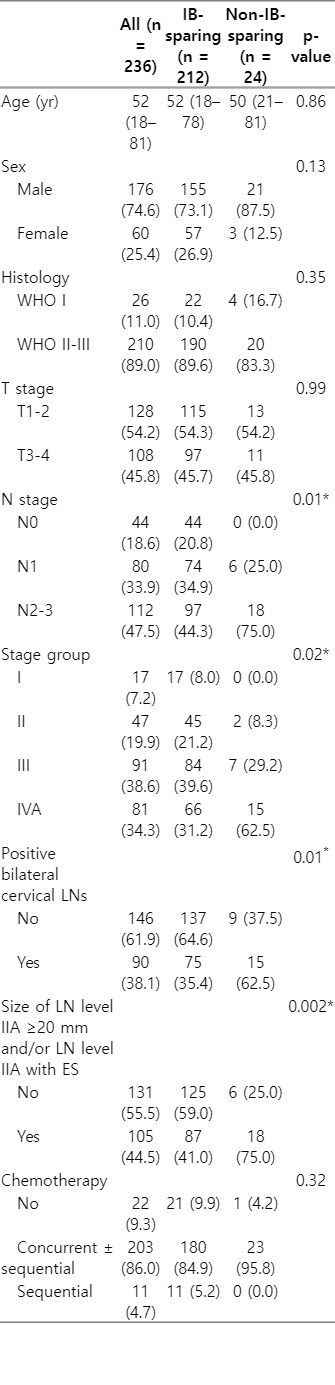
Table 1 shows the characteristics of all included patients. The median age at diagnosis was 52 years (range, 18 to 81 years). There were 176 males (74.6%) and 60 females (25.4%). Most of the patients (n = 210; 89%) had the World Health Organization type II to III NPC. Overall, 54.2% of patients (n = 128) were staged as T1–T2, and 45.8% (n = 108) had stage T3–T4 disease. In total, 18.6% (n = 44) had lymph node-negative disease, and the remainder had node-positive disease (n = 192; 81.4%). In terms of AJCC stage, 17 patients (7.2%) were stage I, 47 (19.9%) stage II, 91 (38.6%) stage III, and 81 (34.3%) stage IV. Overall, four patients (1.7%) had level IB lymph node metastasis at the time of diagnosis. Among these patients, two underwent non-IB-sparing RT, and two underwent unilateral IB-sparing RT. Patients who received non-IB-sparing RT had significantly more advanced N stages and bilateral neck involvement than those who received IB-sparing RT (all p = 0.01). Additionally, the non-IB-sparing RT group had more large (greatest dimension ≥20 mm) level IIA lymph nodes and/or IIA nodes with extracapsular spread than the IB-sparing RT group (p = 0.002). After propensity score matching, all variables in the two groups were well balanced (Supplementary Table S1).
All patients completed RT. Eighteen patients (7.6%) had residual lesions in the nasopharyngeal (n = 11) or neck (n = 4) regions, or both (n = 3). Of these patients (n = 18), four underwent additional lymph node dissection, and two patients underwent repeat RT for residual disease.
2. Treatment outcomes and patterns of failure
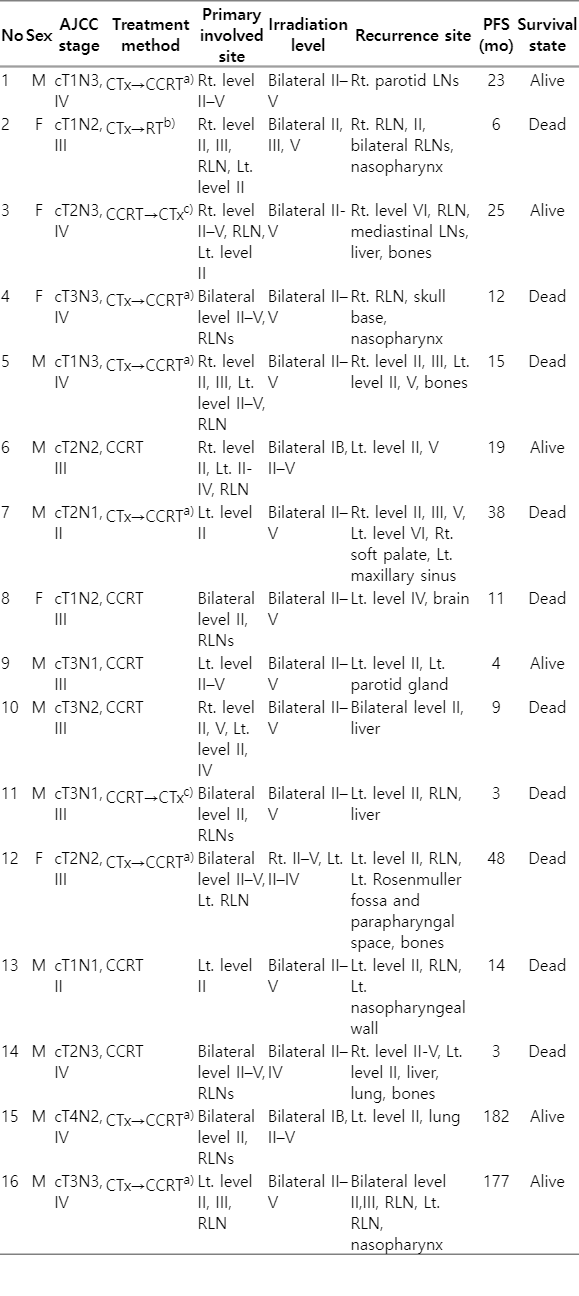
The median follow-up duration was 78 months (range, 7 to 194 months). A total of 67 patients (28.4%) developed recurrence during follow-up. Overall, local recurrence, regional recurrence, and distant metastasis occurred in 11.9% (28/236), 6.8% (16/236), and 16.1% (38/236) of patients, respectively. Of the patients who developed regional recurrences, seven developed isolated regional recurrences, three developed both regional and local recurrences, five developed both regional and distant metastasis, and one developed concurrent locoregional and distant metastasis. Table 2 summarizes detailed information on the 16 patients who experienced regional recurrence. All of these patients were clinically node positive, and N1 and N2-N3 disease accounted for 25% and 75%, respectively. Of these 16 patients, 14 received level IB-sparing RT, and two received non-IB-sparing RT. Regarding recurrence site, 12 had level II lymph nodes, three had level III lymph nodes, five had level IV lymph nodes, three had level V lymph nodes, and seven exhibited metastasis of RLNs. None of these patients showed evidence of level IB recurrence, regardless of level IB lymph node irradiation status.
In the entire cohort, RRFS, LRRFS, PFS, and OS rates at 5 years were 93.8%, 84.5%, 68.6%, and 80.9%, respectively. In the matched cohort, the 5-year rates of RRFS, LRRFS, PFS, and OS were 87.8% and 90.8% (p = 0.95), 66.1% and 77.2% (p = 0.39), 50.9% and 41.3% (p = 0.21), and 70.6% and 61.7% (p = 0.21) in the IB-sparing and non-IB-sparing groups, respectively. IB-sparing RT was not significantly associated with treatment outcomes in the matched cohort, and the Kaplan-Meier curves are illustrated in Fig. 2.
3. Xerostomia and dosimetric outcomes
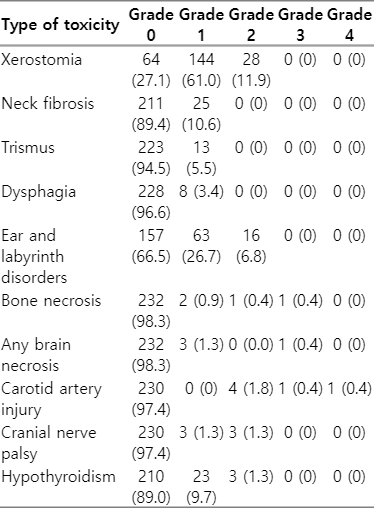
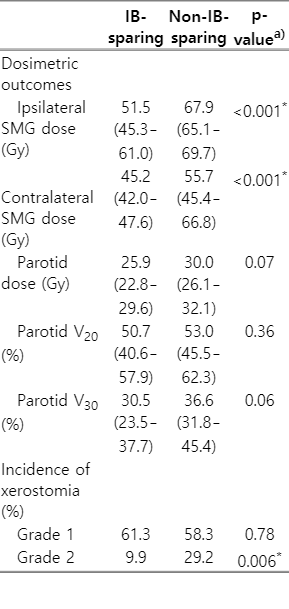
In this study, xerostomia was the most common late toxicity and was observed in 72.9% of the patients (Table 3). Most of these patients had mild symptoms; however, 28 had grade 2 xerostomia (none had grade ≥3). Table 4 shows the dosimetric outcomes and incidence of xerostomia of the two groups according to IB-sparing RT. The median values of ipsilateral (51.5 vs. 67.9 Gy; p < 0.001) and contralateral (45.2 vs. 55.7 Gy; p < 0.001) mean SMG doses of the IB-sparing group (n = 138) were significantly lower than the non-IB-sparing group (n = 17), respectively. The incidences of grade 2 xerostomia were 9.9% and 29.2% in the IB-sparing and non-IB-sparing groups, respectively, and the difference in incidence between these two groups was significant (p = 0.006). However, there were no significant differences in the dosimetric parameters (mean dose, V20, and V30) of parotid glands according to IB-sparing RT.
4. Other toxicities
During RT, 43 patients (18.2%) were admitted for nutritional support owing to acute grade 3 mucositis. The other patients had acute grade 1 to 2 mucositis that did not interfere with oral intake and continued treatment as scheduled. Table 3 lists the late toxicities documented for all patients. Ear and labyrinth disorders, including hearing impairment, ear fullness, or tinnitus, were the second most common late toxicity and were observed in 33.5% of patients (n = 79). Six patients (2.5%) were diagnosed with carotid artery injury, and one underwent urgent intervention due to severe bleeding and subsequently suffered permanent neurological damage. Cranial nerve palsy occurred in six patients (2.5%), and the involved sites were cranial nerves V (n = 1), VI (n = 4), and XII (n = 1).
Discussion and Conclusion
In the nasopharynx, there is an extensive submucosal lymphatic plexus. Like with most other head and neck cancers, lymph drainage from the nasopharynx is mainly to the cervical lymph nodes [18]. NPC has a higher cervical lymph node metastasis incidence than other head and neck cancers [19]. Several studies have reported that 69.5%–86.4% of patients have experienced regional node metastasis at the time of NPC diagnosis. Therefore, regional control is a significant concern in the treatment of NPC [5,20,21]. Li et al. [22] found that, of 165 patients with NPC treated with IMRT, 75% had lymph node metastasis at diagnosis, and regional failure occurred in 7.7% after treatment. Similar to these previous studies, in our study, 81.4% of patients had node-positive disease, and 6.8% experienced regional failure during follow-up after treatment.
Previous studies have reported that level II lymph nodes and RLNs are the most common sites of regional node recurrence of NPC [5,6]. Xue et al. [6] found that regional failure occurred in 6.2% of 275 NPC patients who underwent IMRT, among whom the level II and retropharyngeal regions accounted for 70.6% and 52.9% of failures, respectively. Likewise, in our study, most regional recurrences occurred in level II and RLNs after treatment (75% and 43.8%, respectively). Furthermore, although most patients underwent IB-sparing RT (n = 212; 89.8%), no recurrence occurred at level IB. A meta-analysis based on 13 studies found that skip metastases occurring at levels I or VI were scarce because lymph node metastasis of NPC followed a predictable and ordered pattern [5]. In addition, according to several large-scale studies, 2%–4% of NPC patients have level IB metastasis at the time of diagnosis, and 0%–0.2% of patients experience level IB recurrence after treatment [5,12,13].
Elective nodal irradiation to the level IB lymph nodes in NPC has been controversial, but recent international guidelines and several studies recommend IB-sparing RT except for specific disease conditions [13,23–26]. As IMRT is widely used in head and neck cancer, several studies have reported that IB-sparing RT can be applied to selected patients with NPC [12,13,24,27]. Meanwhile, Zhang et al. [13] found that only three of 1,438 patients treated with IB-sparing IMRT had relapsed at level IB; they suggested that patients with level IIA lymph nodes ≥20 mm in diameter and/or extracapsular spread and/or bilateral cervical lymph node involvement at the time of diagnosis are at “high risk” for metastasis or recurrence to level IB. Additionally, Guo et al. [24] demonstrated that it is safe to perform IB-sparing RT for patients at “high risk” of level IB recurrence if there is no level IB lymph node metastasis and no tumor involvement of the oral cavity or anterior half of the nasal cavity. Indeed, in our study, patients in this high-risk group received significantly more IB-covering RT (i.e., non-IB-sparing RT) than IB-sparing RT; patients who had level IB lymph node metastasis at diagnosis underwent IB-covering RT. Even after propensity score matching, IB-sparing RT had no significant effect on treatment outcomes. Furthermore, none of the patients exhibited level IB recurrence, irrespective of IB-sparing RT. Therefore, our results were consistent with previous other studies, and we reconfirmed that IB-sparing RT is sufficiently safe and feasible for most NPC patients without level IB lymph node metastasis.
RT is an effective treatment modality for head and neck cancers, but RT-related toxicities can significantly affect the quality of life [28]. In NPC, xerostomia is one of the most common side effects of RT, which directly affects the patient's diet and nutrition [13]. Xerostomia is related to the function of the major salivary glands (SMG and parotid glands), and many studies have focused mainly on achieving parotid gland-sparing [29]. Indeed, parotid-sparing IMRT is known to be an effective method in improving salivary flow rate and reducing xerostomia and has been applied to various head and neck cancers; meanwhile, especially in NPC, there are relatively few studies on the relationship between SMG-sparing IMRT and xerostomia [30]. However, xerostomia is closely associated with the total volume of unstimulated saliva and mucins, produced mainly by the SMGs [16]. In oral pharyngeal cancer studies, it has been reported that SMG-sparing RT is an effective treatment modality and is helpful for reducing xerostomia [27,31,32]. Gensheimer et al. [32] found no difference in regional failure among patients with locally advanced oropharyngeal cancer who received contralateral SMG-sparing IMRT compared with non-sparing RT, but there were significant reductions in xerostomia by 6% and 41%, respectively. These results were consistent with our study for NPC patients. After matching, there was no significant difference in treatment outcome between the IB-sparing RT and non-IB-sparing RT groups, but IB-sparing RT effectively lowered the mean SMG doses and significantly reduced the incidence of grade 2 xerostomia. Also, in our results, there was no significant difference in parotid gland dose according to IB-sparing RT, suggesting that xerostomia was more closely related to SMG dose.
Previous studies have demonstrated mean SMG doses ≥39 Gy to cause significant decreases in salivary flow rate and increased incidence of grade 4 xerostomia [33,34]. Since the location of the SMGs is in lymph node level IB, as expected, the median values of the mean SMG doses in our study varied significantly according to IB-sparing status. However, even with IB-sparing RT, the mean SMG doses on the ipsilateral and contralateral sides were 51.2 Gy and 45.2 Gy, respectively, which were higher than the mean dose threshold (i.e., 39 Gy). Nevertheless, there was a 23.4% absolute reduction in the incidence of grade 2 xerostomia in the bilateral IB-sparing RT group compared with the non-IB-sparing RT group. Therefore, our results showed that IB-sparing RT could significantly improve patient-rated xerostomia without compromising disease control.
There were several limitations to this study. First, this was a retrospective study that collected data at a single institution. Therefore, toxicities, including xerostomia, were not fully evaluated due to this study being based on a review of medical records. Second, in this study, xerostomia was not assessed by measuring salivary output. However, some studies have shown a significant correlation between patient-reported xerostomia scores and salivary production [35,36]. Last, level IB elective irradiation was determined by the physician’s clinical judgment. Selective bias toward applying IB-covering RT for patients with more advanced N-stage disease was inevitable. Therefore, we performed a propensity score matching analysis to minimize inherent bias.
In conclusion, our results indicate that elective nodal irradiation excluding level IB lymph nodes is safe and feasible for most NPC patients. Additionally, as an option to reduce the occurrence of grade 2 xerostomia, IB-sparing IMRT may be an effective treatment modality.
Notes
Statement of Ethics
The study protocol was approved by our Institutional Review Board of Seoul National University Hospital (IRB No. 2012-192-1187), which waived the requirement for informed consent due to the retrospective study design.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2019R1F1A1040583) and the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. NRF-2020M2D9A1093990).
Author Contributions
Conceptualization, Kim D, Wu HG. Investigation and methodology, Kim D, Keam B, Ahn SH, Choi CH, Wu HG. Writing of the original draft, Kim D, Wu HG. Writing of the review and editing, Kim D, Wu HG. Formal analysis, Kim D, Wu HG. Data curation, Kim D, Wu HG. Visualization, Kim D, Wu HG.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2022.00346.
Baseline characteristics of the matched cohort