Research progress and treatment of radiation enteritis and gut microbiota
Article information
Abstract
Radiation enteritis is a kind of intestinal radiation injury in patients with pelvic and retroperitoneal malignancies after radiotherapy, and its occurrence and development process are very complicated. At present, studies have confirmed that intestinal microecological imbalance is an important factor in the formation of this disease. Abdominal radiation causes changes in the composition of the flora and a decrease in its diversity, which is mainly manifested by a decrease in beneficial bacterial species such as Lactobacilli and Bifidobacteria. Intestinal dysbacteriosis aggravates radiation enteritis, weakens the function of the intestinal epithelial barrier, and promotes the expression of inflammatory factors, thereby aggravating the occurrence of enteritis. Given the role of the microbiome in radiation enteritis, we suggest that the gut microbiota may be a potential biomarker for the disease. Treatment methods such as probiotics, antibiotics, and fecal microbiota transplantation are ways to correct the microbiota and may be an effective way to prevent and treat radiation enteritis. Based on a review of the relevant literature, this paper reviews the mechanism and treatment of intestinal microbes in radiation enteritis.
Introduction
Radiotherapy is a common and important clinical treatment for abdominal, pelvic, and retroperitoneal malignant tumors. In the process of effectively eliminating tumor cells, the high sensitivity of the intestine to radiation causes local intestinal mucositis and gut microbiota disorder in the irradiation field, thereby forming radiation enteritis [1]. Extensive use of radiation results in radiation damage in up to 75% of radiotherapy recipients [2]. Approximately 90% of patients receiving pelvic radiotherapy had permanent changes in bowel habits after radiotherapy, and 50% experienced a significant reduction in quality of life [3]. At present, with a deeper understanding of the gut microbiota in the occurrence and development of diseases, the mechanism of the gut microbiota in radiation enteritis has become a research hotspot. This article reviews the related studies of intestinal microbes and radiation enteritis, hoping to provide new ideas for improving the toxicity of radiotherapy.
Definition and Role of Gut Microbiota
"Gut microbiota" generally refers to the various microbial communities (bacteria, fungi, archaea, viruses, and protozoan hosts) within the gastrointestinal tract that colonize the intestine. Among these species, the main microbial species in the human intestine include Firmicutes and Bacteroides, followed by Actinomycetes and Verrucomicrobia [4]. The composition of the microbiota is unique to everyone, but it is not fixed. The gut microbiota is affected by the environment, living habits, diet, drugs, and other factors and interacts with the body through the entero-brain axis, entero-liver axis, and entero-lung axis, and it is an important regulator of the body's metabolism and immune system. The interaction among the gut microbiota, host and external environment maintains a balanced state [5]. At the same time, normal gut microbiota has specific functions in host nutrient metabolism, xenobiotic, and drug metabolism, maintaining the structural integrity of the intestinal mucosal barrier, immunomodulation, and resistance to pathogens [6]. However, the gut microbiota are mainly manifested as a symbiotic relationship with the host, and when the intestinal ecological environment changes, it may cause gut microbiota disorders, making these commensal bacteria pathogenic [7].
Definition of Radiation Enteritis and Pathophysiologic Changes
1. Definition of radiation enteritis
Radiation enteritis, which refers to radiation therapy-induced injury to the intestinal epithelium, with or without mild inflammation, is one of the common complications after radiation therapy in patients with pelvic malignancy [8]. Radiation enteritis can be divided into acute radiation enteritis and chronic radiation enteritis according to the time and course of symptoms. Acute radiation enteritis usually occurs during radiotherapy or within 3 months after the initiation of treatment, with the highest incidence being more than 75%, which occurs between weeks 4 and 5. It is usually associated with intestinal dysfunction, and the main clinical symptoms include diarrhea, mucous excretion, urgency of defecation, posterior tenseness and, in rare cases, bleeding [9-12]. The onset of chronic radiation enteritis is relatively late, with the first symptoms generally appearing 9–14 months after radiation exposure, but it can also occur at any time up to 30 years after radiation exposure, with an incidence of approximately 2% to 20%. The symptoms of chronic radiation enteritis are similar to those of acute radiation enteritis, but the bleeding is often more severe [12].
2. Pathophysiological changes in radiation enteritis
Radiation enteritis is caused by the dynamic interaction of intestinal microbiome changes, epithelial cell injury and repair, endothelial cell injury and remodeling, fibroplasia, and enteric nervous system changes. The basic pathological changes include two aspects: intestinal mucosal injury caused by radiation and vascular connective tissue injury caused by radiation vascular endothelial cells [5]. At the initial stage of injury, the proliferation and maturation of intestinal epithelial cells with the ability of rapid division appear abnormal, which leads to reduced mitosis of crypt cells and then leads to thinning of the intestinal mucosa and shortening of the intestinal villi. In addition, there are pathological changes such as telangiectasia, edema, and inflammatory cell infiltration, which eventually lead to intestinal mucosal congestion, edema and exudation, and the clinical manifestations mainly include abdominal distension, abdominal pain, diarrhea, tenesmus, constipation, and bloody stools with mucous. In the later stage, the endothelial cells of the submucosal arterioles of the intestine are swollen, hyperplastic and fibrotic, resulting in the formation of obliterating vasculitis, thereby causing ischemia and hypoxia of the intestinal wall tissue, followed by erosion and ulceration of the intestinal mucosa, hyperplasia of submucosal fibrous tissue, hyaline degeneration of smooth muscle and other pathological changes, finally leading to intestinal wall fibrosis and thickening, intestinal luminal stenosis, etc. This results in intestinal necrosis, intestinal fistula, intestinal perforation, and intestinal obstruction [13,14].
How Radiation Enteritis Affects Gut Microbiota
1. Effects of radiation enteritis on gut microbiota
Derrien et al. [15] compared the gut microbiota of mice in the radio-injured group and the control group, and the results showed that the proportion of Salmonella and Verrucomicrobia in the intestines of the mice in the radio-injured group increased significantly, while the proportion of Firmicutes decreased significantly. Further findings showed that the clinical manifestations of patients were closely related to gut microbiota imbalance. At the same time, Cui et al. [16] determined the relationship between the microbiota of radiation enteritis and radiation sensitivity through 16S rRNA sequencing and found that 6.5 Gy of whole-body radiation gamma radiation changed the community composition of the gut microbiota of C57BL/6 mice. It was further confirmed that the change in gut microbiota affected the survival of mice after radiation, mainly due to the change in the microbiome, which removed the expression of host lncRNAs and made the mice sensitive to radiation. In addition, Johnson et al. [17] surgically exposed the ileum of mice and received a single dose of 19 Gy of high-dose radiation, and found that the number of aerobic bacteria and Lactobacillus were significantly reduced in the gut approximately 2 hours after radiotherapy. Zhao et al. [18] performed a single dose of 10 Gy local high dose precise irradiation on the abdomen of mice, and demonstrated that abdominal radiation disrupted the balance of gut microbiota in mice and significantly reduced the diversity of gut microbiota. In conclusion, the study suggests that abdominal radiation induces gut microbiota dysregulation and reduces the survival rate of irradiated mice in animal models.
Wang et al. [10] analyzed the gut microbiota of 18 patients (the total dose of pelvic radiotherapy was 50.4 Gy in 1.8 Gy/fraction)with cervical cancer complicated with radiation enteritis during radiotherapy and found that the α diversity of the gut microbiota in the patients with radiation enteritis decreased, but the β diversity increased, among which the proportions of Megamonas, Neosphenolipa, and Prevotella increased significantly. Further analysis of the differences in the gut microbiota of the patients before and after radiotherapy showed that the numbers of Coprococcus and Desulfovibrio were significantly reduced after radiotherapy. Manichanh et al. [19] found that patients with radioactive enteritis who had grade 3 or above diarrhea had a significantly higher difference in gut microbiota than those who did not have diarrhea. The gut microbiota remained unchanged in 60% of the patients without diarrhea throughout radiotherapy. However, only 29% of the patients with diarrhea did not have mutations in their gut microbiota. Among them, the number of Bacteroides increased significantly in the gut microbiota of the radioactive enteritis patients with diarrhea symptoms, while Actinomycetes-related bacteria were not detected in the radioactive enteritis patients without diarrhea [20]. Existing research results show that the gut microbiota of patients with radiation enteritis is significantly translocated, the number of Actinobacteriota and Proteobacteria is significantly increased, and many conditional pathogenic bacteria, such as Enterococcus and Enterobacterales, are included.
In summary, the study mentioned above shows that the gut microbiota of the patients with radiation enteritis changes significantly in terms of composition and diversity. In the gut microbiota of the patients with radiation enteritis, the abundance of bacteria belonging to Actinomyces and Proteobacteria increased, and most of these bacteria are conditional pathogenic bacteria. The number of beneficial bacteria (such as Lactobacillus) from Firmicutes and Bacteroidetes decreased significantly. The decrease in the number of beneficial bacteria will promote the proliferation of opportunistic pathogens and promote the release of endotoxins, thus aggravating the intestinal inflammatory response, inducing damage to the intestinal mucosal barrier, and aggravating the disease of patients [21,22].
2. Effects of gut microbiota on radiation enteritis
In recent years, many basic and clinical studies at home and abroad have been conducted on the relationship between gut microbiota and radioactive intestinal injury caused by pelvic radiotherapy, and considerable conclusions have been drawn [22-26].
Gerassy-Vainberg et al. [23] transplanted fecal bacteria from mice in the irradiation group and the control group into germ-free mice and found that the fecal bacteria that was given to the germ-free mice in the irradiation group significantly increased the degree of damage to the intestinal mucosa compared with the fecal bacteria in the control group, and the inflammation score was also significantly increased. When intestinal epithelial cells from both groups were examined separately, the fecal bacteria in the irradiated mice induced a significant increase in interleukin-1β (IL-1β) expression, while the tumor necrosis factor (TNF)-α expression did not change. The fecal flora of the mice in the irradiated group and the control group were cocultured with HT29 human colon cancer epithelial cells. The results showed that the fecal flora of the mice in the irradiated group could significantly increase the expression levels of TNF-α and IL-1β in epithelial cells. To further verify the effect of IL-1β, the researchers added an IL-1β inhibitor to the irradiated mice and found that the degree of damage to the intestinal mucosa in the irradiated mice was significantly reduced compared with that in the control group. This suggests that IL-1β induced by gut microbiota imbalance caused by radiation injury plays an important role in the occurrence and development of radiation enteritis. In recent years, some studies have shown that gut microbiota translocation can affect host metabolism, but the specific mechanism is not clear [24]. At the same time, animal studies have shown that mice treated with fecal transplants have higher survival rates and fewer toxic reactions observed during a 10-day course of radiation compared with conventional mice. This suggests that changing the composition of the gut microbiota can alter the gut's susceptibility to radiation [16].
Chitapanarux et al. [22] administered pelvic radiotherapy at a total dose of 56 Gy in 1.8 Gy in combination with standard treatment of locally advanced cervical cancer with cisplatin 40 mg/m2 per week to patients with FIGO stage IIB–IIIB cervical squamous cell carcinoma, orally administered live Lactobacillus acidophilus and Bifidobacterium bifidum 7 days before and daily throughout radiotherapy in patients with cervical cancer and showed that it reduced the incidence of radiation-induced diarrhea, reduced the use of antidiarrheal drugs, and improved the status of the patients' stools. Ding et al. [25] analyzed patients who had received radiation therapy for cervical and endometrial cancer and had radiation intestinal damage, and the symptoms of radiation enteritis, i.e., diarrhea, rectal bleeding, abdominal pain, and fecal incontinence, were improved in the patients who received fecal microbiota transplantation. Mitra et al. [26] also found in a study of 35 patients with cervical cancer receiving radiotherapy and chemotherapy that the diversity of intestinal microbes in patients was positively correlated with intestinal function during treatment.
Radiation Enteritis Therapy
Currently, the treatment of radiation enteritis mainly includes nutritional support, drug therapy, regulation of intestinal flora, mucosal protection, anti-oxidation, prevention, and treatment of complications. Surgical treatment is required when medical treatment is ineffective (Fig. 1). We summarized the clinical treatment of radiation enteritis [16, 27-33] (Table 1).
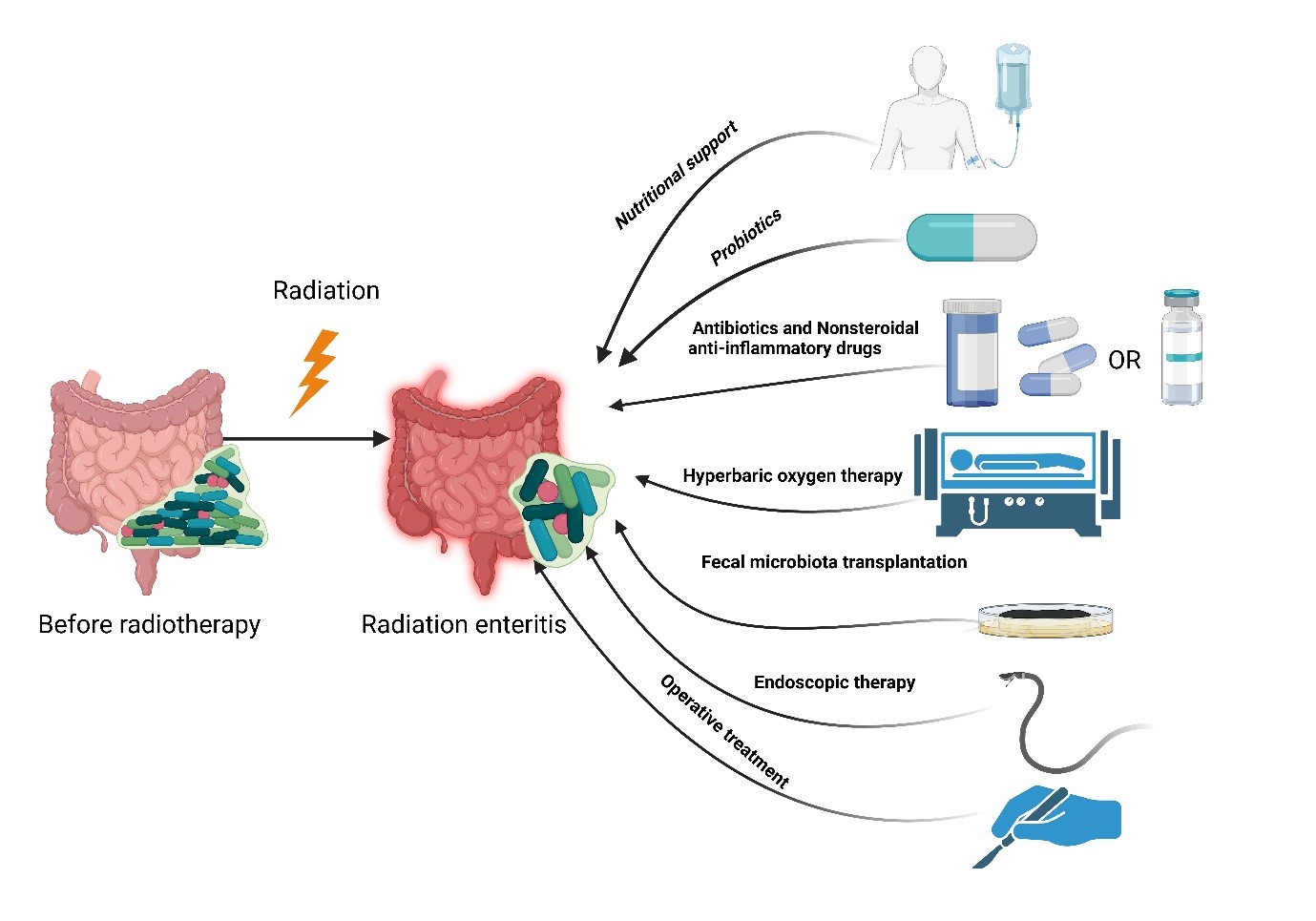
Radiation leads to the reduction of intestinal flora diversity and the treatments are available for radiation enteritis. Created by Biorender.com.
1. Nutritional support
In the treatment of radiation intestinal injury, the value of parenteral nutrition combined with enteral support therapy has been widely recognized. In the early stage of the disease, patients generally have more severe diarrhea or gastrointestinal bleeding, at which time the intestine needs to rest. Therefore, fasting and parenteral nutrition support should be given [27]. The long-term administration of parenteral nutrition to patients can cause intestinal mucosal atrophy. Therefore, when patients have diarrhea and if their gastrointestinal bleeding and other symptoms are under control, they should transition to enteral nutrition in a timely manner. Because the supply of energy to patients through enteral nutrition is in line with the physiological function of the intestine, it is conducive to the repair of damaged intestinal mucosa and epithelial cells, thereby maintaining the barrier effect of the intestinal mucosa and significantly reducing the occurrence of intestinal infections [34].
2. Probiotics, Prebiotics
At present, with further research on gut microbiota, the use of probiotics and prebiotics to treat radiation enteritis has become a research hotspot. Probiotics are a class of living microorganisms, including bacteria and yeast, that play an important role in reducing intestinal damage, reducing the severity of intestinal inflammation, reducing the cell apoptosis rate, increasing lactase, shortening intestinal villi, and so on [29,35]. At the same time, prebiotics are beneficial to the host. Prebiotics are defined as nondigestible, selectively fermented short-chain carbohydrates that allow for specific changes in the composition or activity of the gut microbiota [36]. Probiotics and prebiotics have been widely used in the prevention and treatment of important gastrointestinal diseases such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) and infectious diarrhea [29,37]. Studies have confirmed that probiotics can be used in radiation enteritis, and the use of probiotic preparations such as Lactobacillus acidophilus, Bifidobacterium bifidum and Lactobacillus casei during pelvic radiotherapy resulted in a decrease in the average number of bowel movements and the incidence of diarrhea [22]. Linn et al. [28] conducted a randomized double-blind experiment on 54 patients with cervical cancer with FIGO stage IB and above (who were treated with external pelvic irradiation at a standard dose of 50 Gy with or without synchronous chemotherapy); that is, they were given Bifidobacterium + Lactobacillus acidophilus (experimental group) and placebo (placebo group). The results showed that the incidence of diarrhea, especially moderate to severe diarrhea, was significantly reduced in the experimental group compared with the placebo group, and the severity and days of abdominal pain were also significantly reduced. Although probiotics are effective in the treatment of radiation enteritis, they are still live bacteria and may cause infections in the host. Studies have shown that probiotics have the potential to cause systemic infections [38]. Therefore, attention should be given to dosage and usage when they are used clinically.
3. Antibiotic use
Damage to the intestinal mucosal barrier from abdominal or pelvic radiation therapy can lead to gut microbiota translocation, flora imbalance and bacterial over reproduction, thus causing intestinal infection and aggravating abdominal pain and distension in patients. Pui et al. [39] conducted a randomized controlled trial of colon lavage and oral antibiotics or 4% formalin in patients with chronic hemorrhagic radiation proctitis following radiation therapy. It was found that the enema plus oral antibiotics group not only had improvements in their blood in stool and stool frequency, but this treatment also alleviated diarrhea, stool control ability, and other symptoms. Antibiotic cocktail (ABX) and metronidazole pretreatment were beneficial to the re-speciation of the intestinal microorganisms in irradiated mice. Studies have confirmed that pretreatment with an antibiotic cocktail can effectively reduce intestinal inflammation, prevent intestinal fibrosis, and ultimately improve the survival rate of radiation-induced mice [18]. The above results indicate that antibiotic preconditioning can effectively relieve intestinal microbial disturbance and intestinal injury caused by abdominal radiation.
4. Nonsteroidal anti-inflammatory drugs
Clinically, the anti-inflammatory drugs commonly used to treat radiation enteritis include aminosalicylic acids and steroids. Nonsteroidal anti-inflammatory drugs can effectively inhibit the formation and release of inflammatory mediators to inhibit the intestinal mucosal inflammatory response and reduce intestinal damage, so they are commonly used in the treatment of ulcerative colitis. At present, some research claims that these medications can reduce the symptoms of radiation enteritis. Jahraus et al. [30] conducted a study on 39 prostate cancer patients (the pelvic external beam radiation dose was at least 45 Gy in four fields, and the total tumor dose was at least 64 Gy) receiving pelvic radiotherapy. During the period from 5 days before radiotherapy to 2 weeks after radiotherapy, when balsalazide was taken compared with placebo, intestinal adverse reactions such as diarrhea, dysuria, weight loss, fatigue and nausea were significantly reduced. Kilic et al. [40] conducted a randomized controlled trial showing that sulfasalazine reduced the incidence of radiation enteritis. At the same time, in clinical practice, intestinal adverse reactions were significantly reduced in patients receiving pelvic radiotherapy with sulfasalazine. However, mesalazine and oxalazine were not effective in preventing radiation enteritis. Compared with the control group, mesalazine did not significantly reduce the incidence of radiation enteritis after radiation therapy, and oxalazine even increased the incidence of diarrhea [41,42]. Some studies have shown that glucocorticoids have a certain alleviating effect on radiation enteritis, while some studies have not shown a therapeutic effect in their use. At present, there is still no large sample evidence to confirm their role in the treatment of radiation enteritis. However, in our clinical work, intravenous and topical application of steroids are still used, and they can relieve the symptoms of patients to a certain extent, especially the symptoms of anal pain.
5. Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) is the treatment of diseases by breathing pure or high oxygen levels under high pressure (above normal pressure). HBOT can significantly improve the oxygen supply at the site of intestinal trauma, thereby increasing the oxygen content, oxygen partial pressure and oxygen reserve of radioactive enteritis tissue. HBOT promotes microvascular formation in the normal tissue around the lesion and is the only method that can be considered to increase the number of blood vessels in the irradiated tissue [43,44]. At present, HBOT is also recommended as an adjunct therapy in some of the guidelines for radiation injury treatment of malignant tumors in gynecology and head and neck surgery [45]. HBOT can not only improve the intestinal inflammatory response and promote the healing of the intestinal mucosa but also has a certain preventive effect on radioactive intestinal injury. Yoshimizu et al. [31] reported the efficacy of five patients with radioactive rectal ulcers who received HBOT, all of whom showed significant improvement in symptoms and complete healing of ulcers without adverse reactions. Moreover, for hemorrhagic chronic radiation enteritis, HBOT combined with argon ion coagulation is more effective. Another study retrospectively analyzed 88 patients with radiation injuries, including 22 patients with radiation enteritis, and the average time interval from radiation therapy to the first symptom was 68 months. The subjective parameters of 22 patients before and after HBOT were analyzed, and 15 patients showed improvement (decreased score on the Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic scale). Five patients maintained the same score, and two patients worsened [46]. It is important to note that limitations in the number of hyperbaric oxygen chambers limit the accessibility of this treatment.
6. Fecal microbiota transplantation
After radiotherapy, changes in the type and number of intestinal microbes were associated with radiotherapy injure. Fecal microbiota transplantation (FMT) refers to the transplantation of feces from a healthy donor into the intestines of patients after isolation and culture in vitro to change the composition of their gut microbiota and further affect the digestive, metabolic and immune functions of patients [16]. However, its mechanism of action is still not fully understood. Currently, it is believed that the mechanism may be to restore the disturbed microflora, which can significantly relieve the clinical symptoms of patients and further promote the damage and repair of the intestinal mucosa [47]. FMT is also used in Clostridium/Clostridium difficile infections, inflammatory bowel disease, irritable bowel syndrome, and hepatic encephalopathy [48]. Studies have shown that FMT can relieve symptoms of radiation enteritis and improve gastrointestinal function. Cui et al. [16] transplanted gut microbiota from healthy mice into the intestines of radiation-damaged mice and found that it could increase the gastrointestinal function and epithelial cell integrity of radiation-damaged mice while maintaining the diversity of gut microbiota in irradiated mice. Ding et al. [25] found that three out of five patients with radioactive enteritis who received fecal microbiota transplantation showed significant relief in clinical symptoms and endoscopic manifestations. However, the relief of symptoms could not be maintained, and FMT might be required again. In addition, the study included a small number of cases and no control group. Whether microflora transplantation can be applied to clinical practice in the future still needs to be verified by further clinical studies with large samples.
7. Endoscopic and argon coagulation therapy
Endoscopy and argon coagulation are also commonly used for severe erosion of the intestinal mucosa, ulcers, and intractable hematochezia in radioactive enteritis. In addition, radiofrequency ablation, cryoablation and other treatments have also been reported.
8. Operative treatment
Serious complications such as intestinal obstruction, intestinal necrosis or intestinal perforation may occur when the condition of radioactive intestinal injury progresses to the advanced stage, which will seriously endanger the life and safety of patients. At this time, surgical treatment is the main treatment method. Ruiz-Tovar et al. [33] and Boland et al. [49] showed that approximately one-third of patients with chronic radiation intestinal injury required surgery. More than 50% of patients with severe radiation intestinal injury require surgery to relieve their symptoms.
Conclusion
The gut microbiota is closely related to radiation enteritis, but the current study is too shallow to elaborate its mechanism. More mechanistic studies are needed to provide evidence for alleviating radiation enteritis. While the studies provide interesting insights into the relationship between radiation enteritis and the gut microbiota, some of the limitations of the research should be acknowledged. Of the existing studies, most are based on animal models or small samples of human patients, and lack of control groups in some studies. And moreover, there could be differences between mouse and human gut microbiota. At present, probiotics, antibiotics, and FMT have been used in the clinical treatment of radiation enteritis, but more basic research and clinical trials are needed to evaluate their efficacy and safety. Therefore, in the following study, we will conduct a prospective study on patients with radiation enteritis with different treatment methods and stages.
Notes
Statement of Ethics
As this study did not involve any human subjects, Institutional Review Board approval and informed consent were not required.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
National Natural Science Foundation of China (No.82073339) supported by Luo JD. Changzhou Sci&Tech Program No. CJ20210166 supported by Liu J and CJ20220092 supported by Fang MM. Development Plan of Medical Science and Technology of Shandong province (No. 202209030703) supported by Wu Q.
Author Contributions
Conceptualization, Ren HW, Wu Q. Investigation and methodology, Sun ZQ, Fang MM, Liu J, Sun ZQ, Fang MM, Liu Jun. Project administration, Luo JD and Liu Jun. Resources, Ren HW, Wu Q. Supervision, Luo JD, Sun ZQ. Writing of the original draft, Ren HW. Writing of the review and editing, Luo JD.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.

