A case of cetuximab-induced radiation recall skin dermatitis and review of the literature
Article information
Abstract
Radiation recall presents as an acute inflammatory reaction triggered by systemic therapy, usually chemotherapy, and is typically limited to an area that was previously irradiated. Radiation recall reactions are generally self-limiting and most commonly occur in the skin. Many systemic agents have been described to elicit a radiation recall reaction, but the exact pathogenesis is largely unknown. Here, we describe the first reported case of radiation recall dermatitis following cetuximab. While cetuximab is associated with other skin reactions, oncologists should not exclude radiation recall dermatitis as a potential complication of cetuximab infusion in patients with prior radiation, and special attention should be paid to the pattern of skin changes both in terms of location and chronology.
Introduction
Radiation recall is a rare and poorly understood delayed reaction that occurs in an area of previous radiation exposure in response to the use of systemic anticancer (chemo-, immuno-, or targeted therapies) agents. It most commonly manifests as skin dermatitis (two-thirds of cases) but has also been reported in the lungs or along mucosal surfaces (gastritis, cystitis, or colitis) [1]. Radiation recall dermatitis was first described in 1959 as an inflammatory skin reaction in a previously irradiated area after actinomycin D infusion [2]. The etiology of radiation recall is not well understood. Radiation recall is mostly associated with the use of conventional cytotoxins, such as doxorubicin, gemcitabine, capecitabine, and taxanes [1], but the association of radiation recall dermatitis with cetuximab has not been described before. In this paper, we describe a case of radiation recall dermatitis induced by cetuximab.
Case Report
A 53-year-old man presented in July 2020 with a 2-month history of dysuria, perineal pain, and weak urinary stream. His symptoms did not improve with ciprofloxacin. Cystoscopy found an occlusive mass in the bulbar urethra and biopsy showed invasive moderately differentiated squamous cell carcinoma. Positron emission tomography/computed tomography (PET/CT) showed uptake in a well-delineated lesion in the bulbar urethra as well as a concerning left inguinal lymph node.
The patient received four cycles of paclitaxel, ifosfamide, and cisplatin followed by bulbar urethral resection, left inguinal lymph node dissection, and perineal urethrostomy in April 2021. Pathology showed poorly differentiated invasive squamous cell carcinoma invading the corpus spongiosum and cavernosum with positive margins and five of seven lymph nodes were positive. Pathology also showed extra-nodal extension and the tumor was epidermal growth factor receptor (EGFR)-positive and human papillomavirus-negative. He then completed radiotherapy with concurrent fluorouracil/mitomycin in August 2021. He received 45 Gy in 25 fractions to the elective pelvis and right groin, 50.4 Gy in 28 fractions to the left groin, 59.4 Gy in 33 fractions to the suspected area of extranodal extension in the left groin, and 64.8 Gy in 36 fractions to the area of positive margin in the bulbar urethra (Fig. 1). During his radiation course, he developed grade 3 dermatitis with moist desquamation on the scrotum, the base of the penis, and the left groin, which was treated conservatively until resolution. On first follow-up imaging, a chest CT showed a right middle lobe nodule that was biopsied, revealing metastatic squamous cell carcinoma. He was started on pembrolizumab in November 2021. While on pembrolizumab, he received 30 Gy in 10 fractions to a growing penile lesion in January 2022 and stereotactic body radiation to two enlarging lesions in the left lower lobe of the lung and a right groin nodule in May 2022.
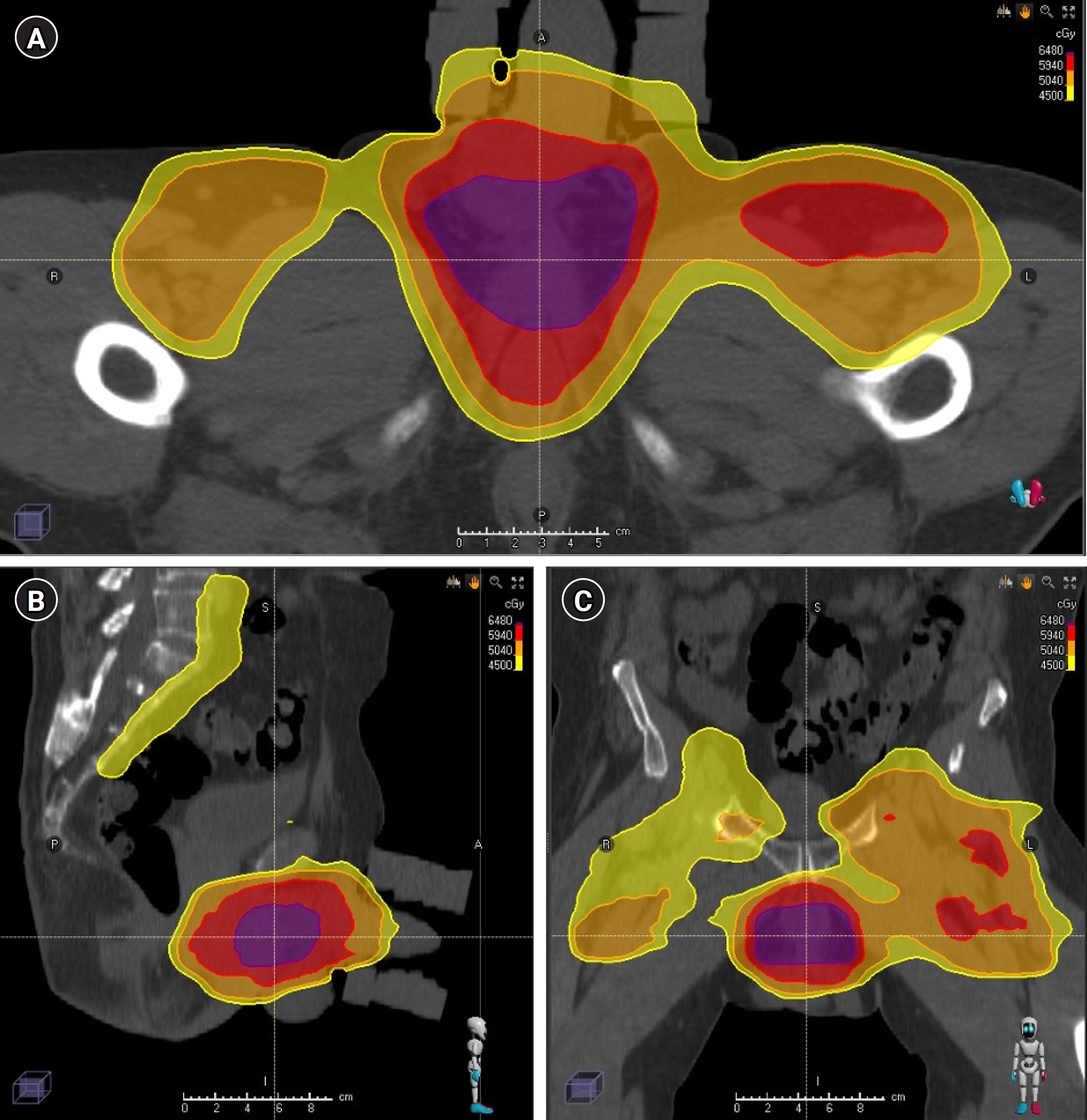
Treatment plan. Representative (A) axial, (B) sagittal, and (C) coronal views of the delivered plan. The prescription dose of 45 Gy (yellow) in 25 fractions to the elective pelvis and right groin, 50.4 Gy (orange) in 28 fractions to the left groin, 59.4 Gy (red) in 33 fractions to the suspected area of ENE in the left groin, and 64.8 Gy (purple) in 36 fractions to the area of positive margin in the bulbar urethra. ENE, extranodal extension.
In June 2022, imaging showed recurrent periurethral mass and in July 2022, he was started on weekly cetuximab. In August 2022, he noted an acneiform rash associated with cetuximab on his face and chest for which he was seen by a dermatologist and prescribed topical clindamycin that was subsequently switched to topical BenzaClin (Fig. 2). On cycle 7 of cetuximab, he reported significant scrotal and groin erythema and moist desquamation (Fig. 3). These skin findings were morphologically distinct from the acneiform rash on his face and chest and were similar to the skin toxicity he experienced during his course of radiation 1 year prior. Radiation recall was suspected, and the skin was managed conservatively. The patient returned for a skin check 3 days later with decreased erythema and improving moist to dry desquamation. The dermatitis completely resolved within 2 weeks and cetuximab was resumed without delay.
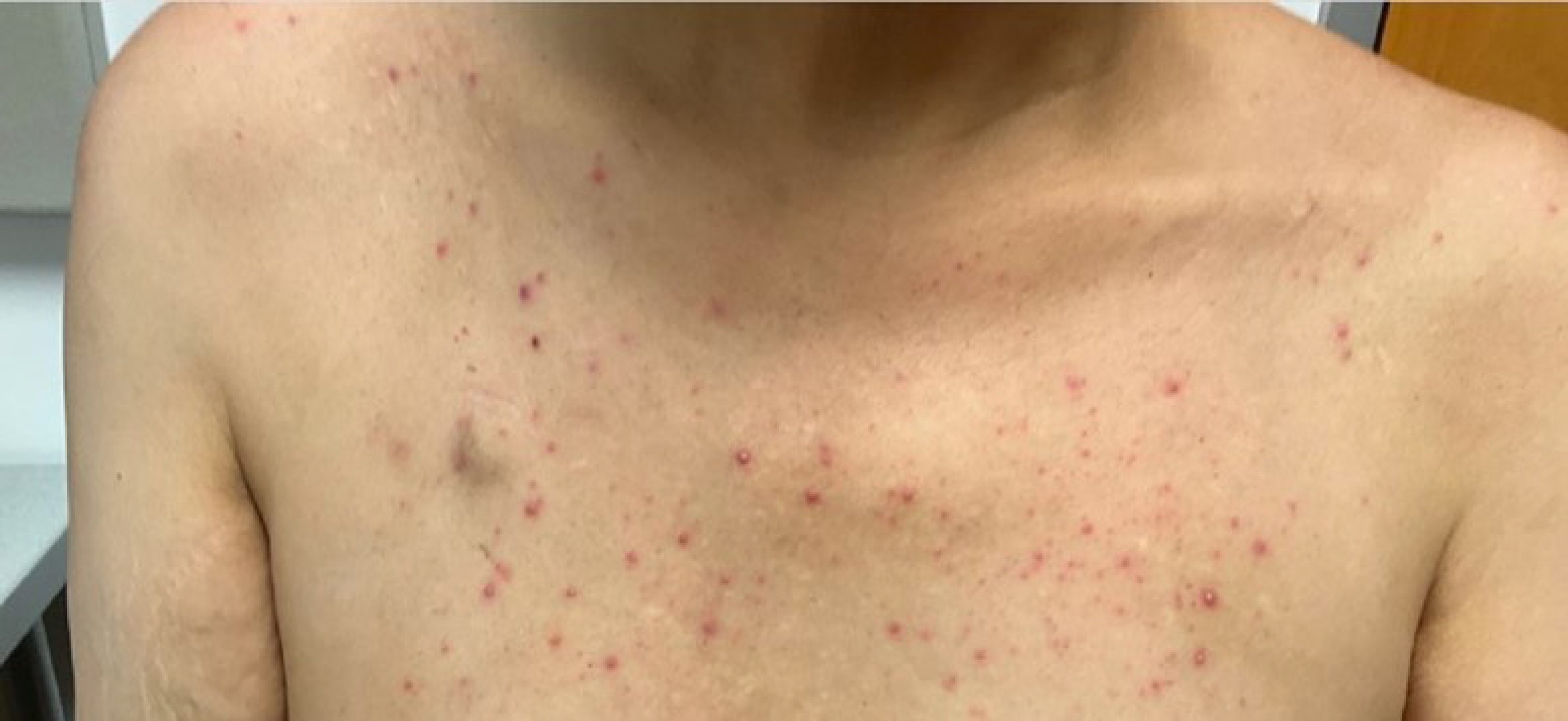
Generalized cetuximab-associated skin rash. The patient experienced a papulo-pustular eruption during cetuximab infusions distinct from the skin toxicity seen in the groin.
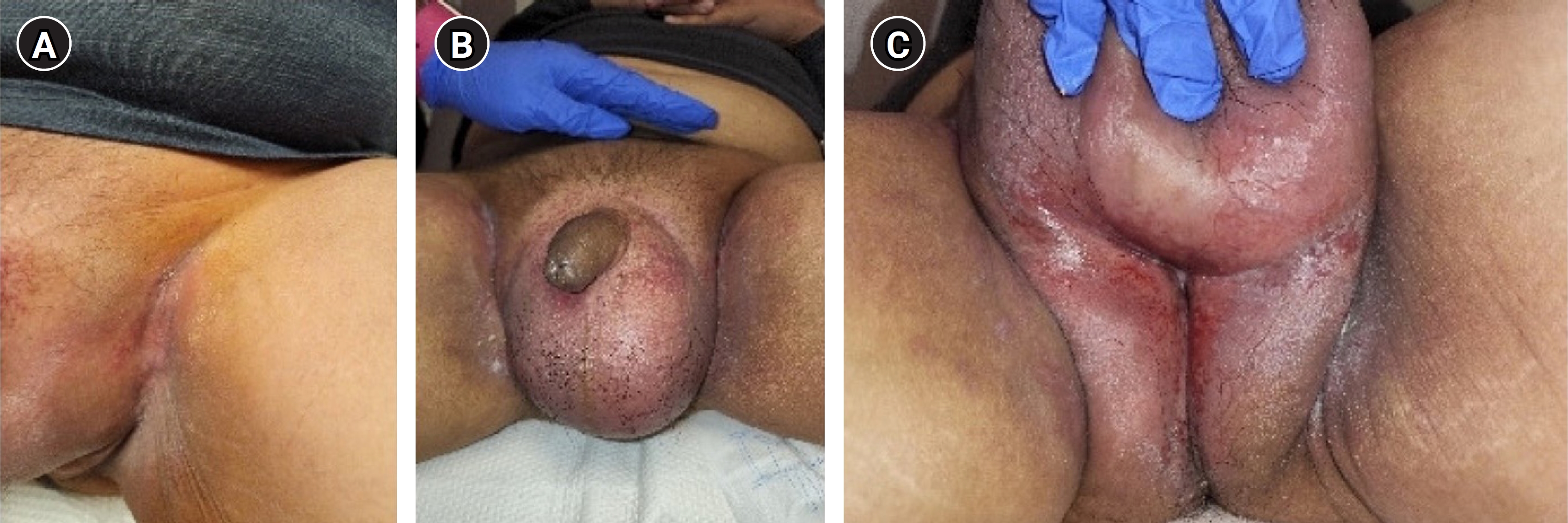
Radiation recall skin reaction during cetuximab treatment. (A) Desquamation and erythema in left inguinal skin fold. (B) Scrotal swelling and erythema. (C) Wet desquamation and erythema of posterior scrotum and perineum.
The informed consent was waived.
Discussion
To our knowledge, this is the first reported case of radiation recall dermatitis with cetuximab. The true incidence of radiation recall is unknown likely due to the fact that several factors influence the development of radiation recall including systemic agent selection and dose, radiation dose and location, and the time between the end of radiation and administration of systemic therapy. Radiation recall reactions have historically been reported with conventional cytotoxic agents such as doxorubicin [3], actinomycin-D [2], bleomycin [4], capecitabine [5], paclitaxel [6], and dactinomycin [3]. It has also been reported with tamoxifen [7], trastuzumab with vinorelbine [8], pemetrexed [9], gefitinib [10], and bevacizumab with gemcitabine [11]. More recently, cases of radiation recall associated with other targeted and immunotherapeutic agents have been also reported [1].
While many systemic or topical agents have been associated with radiation recall, the exact relationship between radiation dose and recall is not clear with some reports suggesting low-dose threshold of around 18 Gy [12] and others suggesting higher threshold dose (40 Gy) [4]. It is likely that the systemic or topical agents also play a role in determining the radiation threshold dose. Timing and schedule of systemic therapy may also play a role. Some series have reported a radiation recall reaction after the first dose of a systemic therapy following radiation [13], while other reported instances of radiation recall only until the second infusion [12], raising the question of a requisite “threshold dose” to cause the radiation recall reaction. Analysis of the MammoSite breast brachytherapy registry trial was done to evaluate the frequency of radiation recall reactions and the impact of timing between radiation and systemic therapy. The majority of patients in this cohort (75%) received doxorubicin-based chemotherapy [14]. The rate of radiation recall reactions in patients who received chemotherapy within 3 weeks after the completion of radiation was 18% (9/50 patients) compared to 7.4% (6/81 patients) in patients who started adjuvant chemotherapy more than 3 weeks after completion of radiation [14]. This trend was not statistically significant (p = 0.09).
Several hypotheses have been posed related to the pathogenesis of radiation recall reactions, but they lack supportive evidence. One hypothesis is that radiation alters vascularization in normal tissue due to endothelial cell damage and capillary proliferation which may affect the pharmacokinetics of drug delivery leading to the recall phenomenon [7]. Others have suggested that stem cells in a previously irradiated area have DNA damage or altered biology, including increased proliferation, which could lead to increased sensitivity when exposed to subsequent chemotherapy [15]. These hypotheses are based on some of the early work characterizing the effect of radiation on epithelial stem cells leading to late effects of radiation by Hellman and Botnick [16] and the proposed biological changes in stem cell populations after radiation by Seymour et al. [17]. Given the fact that a rechallenge after a radiation recall reaction does not always redemonstrate the phenomenon and the fact that cytotoxic and non-cytotoxic drugs have been shown to produce the radiation recall reaction, further work into the mechanism and pathogenesis of radiation recall is needed [15].
Cetuximab is a chimeric monoclonal antibody that binds to and inhibits EGFR. To our knowledge, radiation recall dermatitis after cetuximab has not been described before. Cetuximab-induced radiation recall mucositis has been reported in one patient in a case series analyzing targeted agents [18]. The patient had head and neck cancer that was treated with radiation over 20 years prior to the administration of cetuximab and developed mucositis 7 weeks after cetuximab infusion. Interestingly, our patient also developed a dermatitis reaction on week 7 of cetuximab. In a different report, a 70-year-old male with squamous cell carcinoma of the tonsil was treated with radical neck dissection, adjuvant radiation, and weekly cetuximab, with grade 3 skin toxicity during radiation. One year later, he was found to have metastasis and was treated with carboplatin and gemcitabine. Skin ulceration developed in the area of prior skin reaction in the radiation fields [19]. While the report attributes this radiation recall reaction to the initial cetuximab treatment, it is more likely that this skin reaction is related to gemcitabine. Unlike cetuximab, reports of radiation recall dermatitis have been described with the use of erlotinib, an EGFR inhibitor [20].
The most recent version of the US National Cancer Institute Common Terminology Criteria for Adverse Events (v.5, 2017) lists “radiation recall reaction (dermatologic)” as a distinct entity defined as “a finding of acute skin inflammatory reaction caused by drugs, especially chemotherapeutic agents, for weeks or months following radiotherapy. The inflammatory reaction is confined to the previously irradiated skin and the symptoms disappear after the removal of the pharmaceutical agent,” and is classified in five grades as below: (1) grade 1, faint erythema or dry desquamation; (2) grade 2, moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema; (3) grade 3, moist desquamation in areas other than skin folds and creases; bleeding induced by minor trauma or abrasion; (4) grade 4, life-threatening consequences; skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site, skin graft indicated; and (5) grade 5, death.
Treatment of radiation recall dermatitis is usually supportive with wound management and cessation of the suspected drug needed in high-grade cases. Therefore, the identification of the inducing agent is crucial. Cetuximab however is associated with other skin reactions, such as most commonly papulopustular acneiform dermatitis, xerosis or dry/flaky skin, or pruritis. In this case, the patient experienced two morphologically distinct skin reactions, both more classic cetuximab-associated acneiform dermatitis on the chest and radiation recall dermatitis localized to the groin with distinct morphology as shown in Figs. 2 and 3. Oncologists should not exclude radiation recall dermatitis as a potential complication of cetuximab infusion in patients with prior radiation, and special attention should be paid to the pattern of skin changes both in terms of location (localized to a prior radiation field or more diffuse or unrelated to prior radiation field), morphology of the skin changes, and chronology.
Notes
Statement of Ethics
This study was conducted and approved by University of California, San Francisco Institutional Review Board (No. 20-31257).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author Contributions
Conceptualization, Sabol RA, Patel AM, Sabbagh A, Wilson C, Yuen F, Lindenfeld P, Aggarwal R, Breyer B, Mohamad O. Investigation and Methodology, Sabol RA, Patel AM, Sabbagh A, Wilson C, Yuen F, Lindenfeld P, Aggarwal R, Breyer B, Mohamad O. Writing of the original draft, Sabol RA, Patel AM, Sabbagh A, Mohamad O. Writing of review and editing, Sabol RA, Patel AM, Sabbagh A, Wilson C, Yuen F, Lindenfeld P, Aggarwal R, Breyer B, Mohamad O. All authors have proofread the final version.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
