The location of locoregional recurrence in pathologic T3N0, non-irradiated lower rectal cancer
Article information
Abstract
Purpose
To investigate the patterns of locoregional recurrence of pathologic T3N0 (pT3N0) lower rectal cancer omitting postoperative radiotherapy (RT) and explore the potential of modification of a RT field.
Materials and Methods
From Jan 2003 to Nov 2011, 35 patients omitting preoperative or postoperative RT for pT3N0 lower rectal cancer were included. We defined the lower rectal cancer as the tumor with the inferior margin located below the virtual line-a convergent level between rectal wall and levator ani muscle. All patients had radiologic examinations for recurrence evaluation during the follow-up duration.
Results
The median follow-up duration was 66.4 months (range, 1.4 to 126.1 months). Eight (22.9%) of the 35 patients had recurrence. Three (8.6%) was local recurrence (LR) only, 3 (8.6%) was distant metastasis (DM) only, and 2 (5.7%) was LR with DM. All LR were located at primary tumor sites. The overall survival rate, LR-free survival rate, and DM-free survival rate at 5 years was 79.8%, 83%, and 87%, respectively. All LR developed from tumors over 5 cm. However, there was no statistical significance (p = 0.065). There was no other risk factor for LR.
Conclusion
Even though the patients included in this study had pathologically favorable pT3N0 rectal cancer, LR developed in 14.3% of patients. Most of the LR was located at primary tumor sites prior to surgery. Based on these findings, it might seem reasonable to consider postoperative RT with a smaller radiation field to the primary tumor site rather than the conventional whole pelvic irradiation.
Introduction
The Dutch colorectal cancer group showed the benefits of preoperative short course radiotherapy for the local control of rectal cancer [1]. Also, phase III studies such as the German Rectal Cancer Group trial and National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 trial reported that preoperative concurrent chemoradiotherapy (CCRT) could reduce the pelvic recurrence better than postoperative CCRT [2,3]. After these trials, there was a shift of paradigm from the postoperative to preoperative CCRT for rectal cancer. The current standard of treatment for locally advanced rectal cancer is preoperative CCRT followed by surgery and postoperative chemotherapy. However, most of these trials included the patients with heterogeneous stage II/III rectal cancer and could not find a benefit from the CCRT for specific subgroups.
Gunderson et al. [4] separated the patients with rectal cancer into three risk groups on the basis of differential relapse and survival rates. Reports indicated that the patients with T3N0 rectal cancer had intermediate risk: relatively lower relapse rate and higher survival rate and the postoperative chemoradiotherapy (CRT) after curative resection could not improve the clinical outcome for patients with intermediate risk of rectal cancer. The National Comprehensive Cancer Network (NCCN) guidelines, however, still recommend the preoperative CRT for the patients with cT3N0 rectal cancer.
Recently, with the advanced diagnostic radiologic technique, the accuracy increment of prediction of pathologic T stage using magnetic resonance imaging (MRI) and endorectal ultrasonography has been achieved. Some of the surgeons conduct surgery first on the basis of radiologic findings in cT3N0, and do not follow the NCCN guidelines. According to the European Society of Medical Oncology (ESMO) guidelines, cT1-2 and cT3 rectal cancer with perirectal tissue invasion <1-5 mm on the MRI and with negative circumferential resection margin (CRM) can be undertaken by surgery first [5].
After the curative resection without any neoadjuvant treatment, we can consider chemotherapy alone for patients with proximal pT3N0 rectal cancer with negative margins and favorable pathologic features. Except in these patients, adjuvant radiation therapy (RT) is still recommended. Since the total mesorectal excision (TME) has been widely used, both local control and disease specific survival have been improved. Some surgeons believe that surgery alone is enough to treat the intermediate risk of rectal cancer in the era of standardized TME. The reasons for omitting RT are to get a more precise pathologic stage, to avoid over-treatment, to reduce RT-induced complications, and to preserve the quality of life. The information of RT-induced toxicities is on the basis of the European studies that compared preoperative RT and TME. They reported more late toxicities like bowel and sexual dysfunctions after RT compared with TME alone [6]. If the fibrosis of normal tissue and the damage of blood vessels and nerves can be decreased by reducing the RT volume, RT-induced complications can also be decreased.
Overall, we could not demonstrate the role of postoperative RT in pT3No rectal cancer with this study. The aim of this study was to investigate the patterns of locoregional recurrence (LRR) in patients with pT3N0 lower rectal cancer omitting postoperative RT. We focused on the location of local failure in order to explore the potential of modification of postoperative RT target volume.
Materials and Methods
1. Patients
From Jan 2003 to Nov 2011, a total of 673 patients who had undergone surgery for pT3N0 rectal cancer were identified. Of them, 182 patients received preoperative RT or CRT and 299 patients received postoperative RT or CRT. Postoperative RT was omitted in 192 patients. Of 192 patients, 35 patients who had omitted preoperative or postoperative RT for pT3N0 lower rectal cancer were included. All patients had computed tomography (CT) or MRI preoperatively. Medical records, radiological images, and radiological reports of these patients were reviewed. All the patients included in this study received R0 resection. Surgeons were well trained in TME technique and applied the technique to the patients in this study. Omission of postoperative RT was determined by the surgeon's preference. Among the 35 patients, 25 patients (71.4%) received adjuvant chemotherapy.
2. Definition of primary tumor location
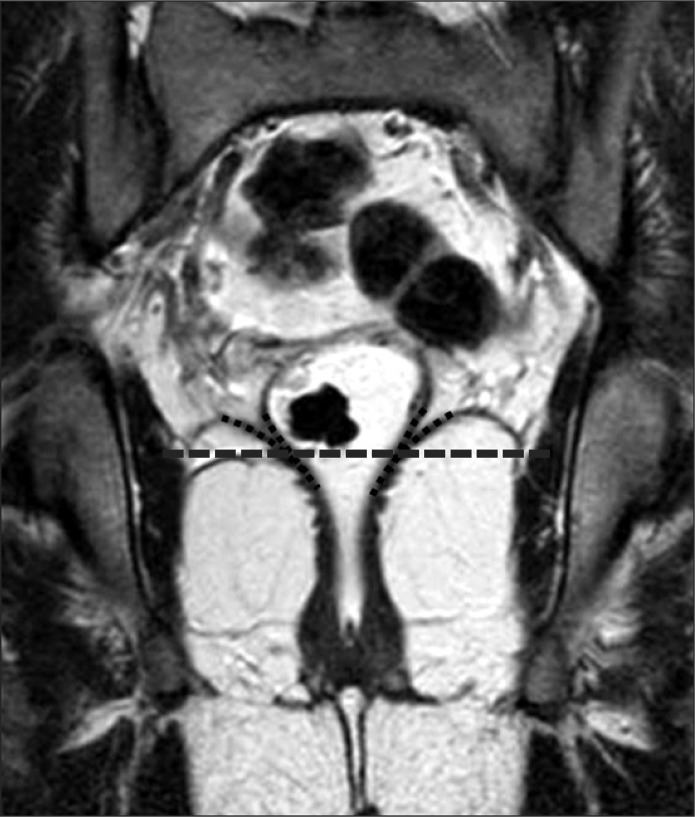
Primary tumor location was determined by inferior tumor margin based on the virtual line (VL). The VL was a convergent level between rectal wall and levator ani muscle. If the inferior margin of tumor was located below the VL, it was defined as a lower rectal tumor (Fig. 1).
3. Evaluation of recurrence
All patients had radiologic examinations for recurrence evaluation during the follow-up duration and were followed by a serial clinical examination and carcinoembryonic antigen assessment. Recurrent tumor that could not be explained by normal or postoperative changes at anastomotic sites and regional metastatic lymph node recurrence documented by colonoscopic, radiologic, or pathologic examination was defined as local recurrence (LR). Recurrent disease in any other location was defined as distant metastasis (DM). Calculation of LR included patients who developed LR only and patients who developed both LR and DM. Cumulative events of LRs were counted. Overall recurrence was considered when patients developed either LR or DM. In case of recurrence, radiologic examinations were compared with initial preoperative CT or MRI to evaluate recurrent tumor locations.
4. Statistical analysis
SPSS ver. 20 (IBM, Armonk, NY, USA) was used for statistical analyses. The univariate influence of prognostic factors on LR, recurrence-free survival, and overall survival was analyzed with the Kaplan-Meier method. The p-values < 0.05 were considered statistically significant.
Results
1. Clinical and pathological characteristics
A total of 35 patients were included. Patient and tumor characteristics are listed in Table 1. The median follow-up duration was 66.4 months (range, 1.4 to 126.1 months). The follow-up duration in 32 patients (91.4%) was over 2 years. Median age was 65 years (range, 27 to 81 years). Median preoperative carcinoembryonic antigen (CEA) was 2.8 ng/mL (range, 0.0 to 35.0 ng/mL) and median postoperative day 7 CEA was 1.3 ng/mL (range, 0.0 to 11.9 ng/mL). Twenty-two patients (62.9%) had undergone lower anterior resection. Abdominoperineal resection (APR) and Hartmann's operation was performed in 34.3% and 2.9%, respectively. The median number of dissected lymph node was 18 (range, 6 to 45). In 8 patients (22.9%), the number of dissected lymph nodes was under 12. All operations for included patients were R0 resection. Median resection margin (RM) was 1.5 cm (range, 0.1 to 15.0 cm) in distal, 12 cm (range, 2.5 to 30.0 cm) in proximal, and 0.6 cm (range, 0.1 to 1.3 cm) in circumferential. There was 13 patients with close RM; 10 patients with distal RM < 1 cm; 3 patients with CRM < 0.2 cm. Of them, 9 patients received postoperative chemotherapy. Among the 4 patients who did not receive postoperative chemotherapy, 1 patient refused adjuvant treatment and others did not received adjuvant treatment by surgeon's opinion. Median tumor size was 5 cm (range, 2.5 to 9.0 cm). Poorly differentiated adenocarcinoma was found only in 1 patient. Lymphovascular invasion (LVI) was identified in 11.4% and there was no perineural invasion (PNI). Most of the patients had favorable histologic features.
2. Patterns of recurrence
Eight (22.9%) of the 35 patients developed recurrences (Table 2). Three (8.6%) was LR only, 3 (8.6%) was DM only, and 2 (5.7%) was LR with DM. The mean time to recurrence was 89.9 months. All LR was located at primary tumor sites (Fig. 2). DMs were observed in lung (3 patients), liver (1 patient), and para-aortic lymph node (1 patient).

Location of local recurrence. Computed tomography or T2-weighted magnetic resonance images of 5 patients who had local recurrence. Left 2 images of each patient show primary rectal cancer (white arrow, 1A, 1B, 2A, 2B, 3A, 3B, 4A, 4B, 5A, and 5B) in the lower rectum. Right 2 images of each patient show recurrent rectal cancer at primary tumor site (white arrow head, 1C, 1D, 2C, 2D, 3C, 3D, 4C, 4D, 5C, and 5D).
3. Risk factors and survival
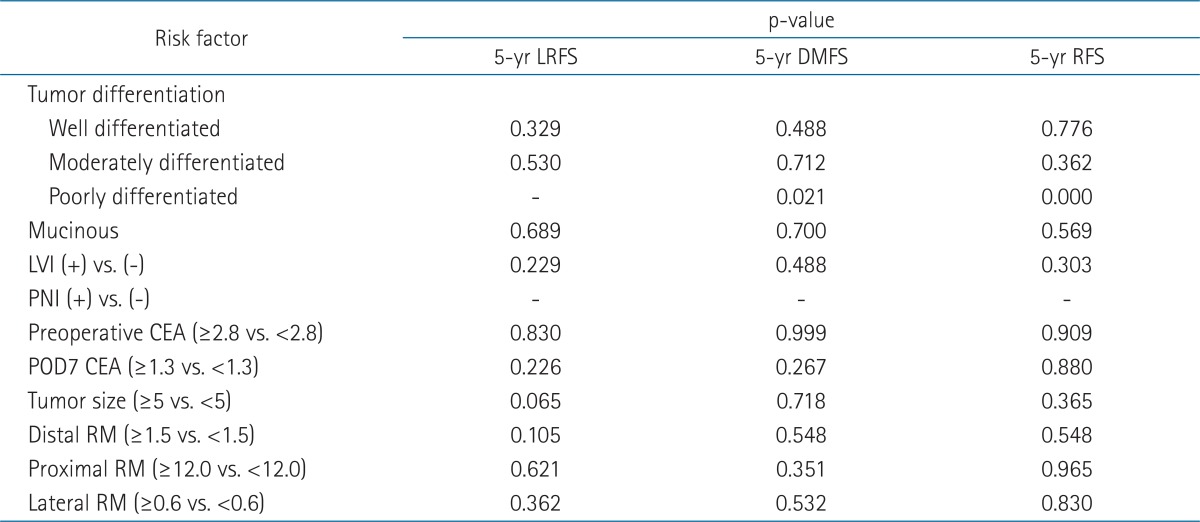
For risk factor analysis, tumor differentiation, LVI, PNI, preoperative CEA, postoperative CEA, tumor size, and RMs (distal, proximal, circumferential) were evaluated (Table 3). There was no LR from tumors smaller than 5 cm. Tumors over 5 cm tend to be related with LR. However, it was not statistically significant (p = 0.065). Although there was no statistical significance, all LRs were developed from the tumors with circumferential resection margins less than 0.6 cm. Poorly differentiated adenocarcinoma seemed to be related with DM (p = 0.021), but there was only 1 patient with poorly differentiated adenocarcinoma and this patient had DM. Therefore, we did not interpret that there was a true relationship between poorly differentiated adenocarcinoma and DM. The methods of operation were not associated with LR. There was no other risk factor for LR. Among 3 patients with LR, 2 patients died despite salvage treatment and 1 patient was alive with disease after salvage CRT. The overall survival rate, local control rate, and DM-free survival rate at 5 years was 79.8%, 83%, and 87%, respectively.
Discussion and Conclusion
The aim of this study was not to find out the role of postoperative RT in lower rectal cancer. In this study we wanted to find out the possibility of modification of the postoperative RT target volume through evaluation of the patterns of LRR to reduce RT-induced toxicities.
Since the introduction of TME, LR rates have been reduced significantly. The intermediate risk group for rectal cancer (pathologic T3N0, T1/2N1) has a favorable outcome. The necessity of RT for patients with intermediate risk of rectal cancer is controversial. Gunderson et al. [7] suggested that postoperative CRT for all patients with intermediate risk of rectal cancer may be excessive. In rectal cancer pooled analysis, there was no statistically significant difference in local failure between patients with surgery plus chemotherapy and with surgery plus CRT [8]. Park et al. [9] evaluated the effect of postoperative RT on LR in stage IIa rectal cancer. They analyzed 390 patients with stage IIa rectal cancer treated with TME plus chemotherapy or CRT. The LR rate was very low (2.8%). RT had no effect on LR and LR-free survival rate. Frasson et al. [10] analyzed the LR, disease-free survival, and cancer specific survival for the patients who had cT3N0/+ or cT2N+ rectal cancer and were not treated with preoperative CRT. They reported the LR rate was 5.4% in the patients with preoperative free CRM (free margin >2 mm from mesorectal fascia) after TME alone. For these patients, they suggested TME alone to avoid overtreatment. However, the current standard of treatment for intermediate risk of rectal cancer is preoperative CCRT followed by surgery. According to the NCCN guidelines, when pT3N0 rectal cancer is diagnosed after upfront surgery, postoperative CCRT is recommended except in proximal rectal cancer with favorable pathologic features. We did not focus on the role of postoperative RT in pT3N0 rectal cancer. Even though the rate of LRR is low, we evaluated the patterns of LRR to find out the potential of modification of the postoperative RT target volume for reducing RT-induced toxicities. We found that most of the LRR occurred at the primary tumor site.
The main reason for the controversy about postoperative RT is RT-induced toxicity. Dahlberg et al. [11] studied the functional result after RT with the patients in the Swedish Rectal Cancer trial. They had the patients fill out a questionnaire. Median bowel frequency per week was higher in the irradiated group than the surgery-alone group (p < 0.001). Incontinence for loose stools (p < 0.001), urgency (p < 0.001), and emptying difficulties (p < 0.05) were all more common in the irradiated group.
RT-induced complications in the patients who were included in a large study for comparing preoperative RT plus TME, and TME alone were also reported. Marijnen et al. [12] reported the acute perineal complications were slightly increased in the patients who received APR. Peeters et al. [6] reported late bowel dysfunction and sexual dysfunction increased in the patients with preoperative RT plus TME than TME alone.
Recently, Wang et al. [13] reported the results of conventional RT-induced complications. In their study, incidence of grade 3 lower digestive tract toxicities was 30.8% and grade 3 urinary toxicities occurred in 2.1% of patients at 6 weeks after completion of RT. Late complications developed in 19.1% of patients (grade 1/2 lower digestive tract toxicities, 17%; grade 1/2 urinary toxicities, 2.1%).
In a systemic review and meta-analysis of the impact of preoperative RT on long-term functional outcome, the majority of studies reported higher rates of anorectal dysfunction such as fecal incontinence, more bowel movements, higher rate of pad wearing, and urgency after preoperative RT [14].
Therefore, studies have been undertaken to reduce RT-induced toxicity and to modify the RT field. Nijkamp et al. [15] analyzed the locations of LR in the 94 patients included in the Dutch TME trial using the three-dimensional model. In their study, most of the LRs were located at the lower two-thirds of the pelvis. Also, patients without primary nodal involvement and a negative CRM had LR caudal to the S2-S3 interspace. Thus, they suggested that the cranial border of the clinical target volume (CTV) could safely be lowered for patients without primary nodal or CRM involvement. If the cranial border of the CTV is lowered to S2-S3 interspace, the absolute small bowel exposure can be reduced over 60% with three-field conventional RT and 80% with Intensity-modulated radiation therapy.
Syk et al. [16] reported the location of recurrence was related with initial tumor height. Fifty-nine percent of recurrence in patients with the middle and high primary tumor (>5 cm from the anal verge, upper two-thirds of the rectum) was seen in the anastomotic site. Recurrences in patients with the lower primary tumor mostly developed in the lower one-third of the pelvis and were distributed equally to anastomotic site, pre-sacral area, and pelvic side wall. They suggested the lowering of the upper limit of the CTV from 1.5 cm above the promontory to 3.5 cm below the promontory. In their study, LR was in the lower three-fourths of the pelvis for all patients. In this study, using radiologic findings, we defined the locations of tumors according to the VL and compared the locations of recurrences. The LRs in patients with lower tumors were located at the primary tumor site.
In EORTC 22921 trial [17], the RT field was limited the mesorectum below to the S2-S3 interspace. The RT field included 5 cm above and 5 cm below the tumor in longitudinal axis, and extended 3 cm anterior and lateral to the tumor. However, this limited volume is consistent with local control those were reported in previous studies.
Most of these studies investigated the modification of RT target volume with the patients who received preoperative CCRT or preoperative short course RT. We evaluated the locations of local failure in the patients with favorable pathologic features who received surgery first. All LR occurred at the primary tumor location. We suggest that a smaller RT field can be applied to pT3N0 lower rectal cancer patients to reduce the RT-induced toxicities.
There were limitations for statistical analyzing the relationship between LR and the risk factors such as tumor size, methods of operation and RM since this study was a retrospective study with small patients and there were only 5 LR. This study could not reflect the entire T3N0 rectal cancer patients. The patients included in this study had favorable pathologic features. There is a selection bias because receiving surgery first was determined by surgeons dependent on the clinical stage. Since the T3N0 group is very heterogeneous, the ESMO guidelines recommend risk-adapted treatment using the TNM classification with sub-classifications regarding the depth of perirectal tissue invasion. We tried to collect the information of perirectal tissue invasion. However, because 20% (7 patients) of patients preoperatively underwent CT without MRI, the depth of perirectal tissue invasion could not be considered. In this study, we defined the lower rectal cancer as the tumor with the invasion below the convergent level of levator ani muscle and rectal wall. So, even though the tumor located in the lower rectum and was pathologically T3, appropriate RM could be achieved with anal preserving surgery in most cases. There were 3 patients who had tumor near the anal canal with internal sphincter invasion. They received Miles' operation.
The recurrence rate could be underestimated, since this study was retrospective and short follow-up duration. Even though the patients who were included in this study had pathologically favorable pT3N0 rectal cancer, LR developed in 14.3% of patients. Most of the LR was located at primary tumor sites prior to surgery. Based on these findings, it might seem reasonable to consider postoperative RT with a smaller radiation field to primary tumor sites rather than the conventional whole pelvic irradiation.
Notes
No potential conflict of interest relevant to this article was reported.