Clinical outcomes in patients treated with radiotherapy after surgery for cervical cancer
Article information
Abstract
Purpose
The purpose of this study was to analyze clinical outcomes from cervical cancer and stratify patients into risk groups for prognostic factors for early-stage disease.
Materials and Methods
We retrospectively reviewed patients with stage IB or IIA cervical cancer treated with adjuvant radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) following primary surgery at Samsung Medical Center from 2001 to 2011. Adjuvant RT was added for patients with intermediate-risk factors, and adjuvant CCRT was performed on high-risk patients after surgery.
Results
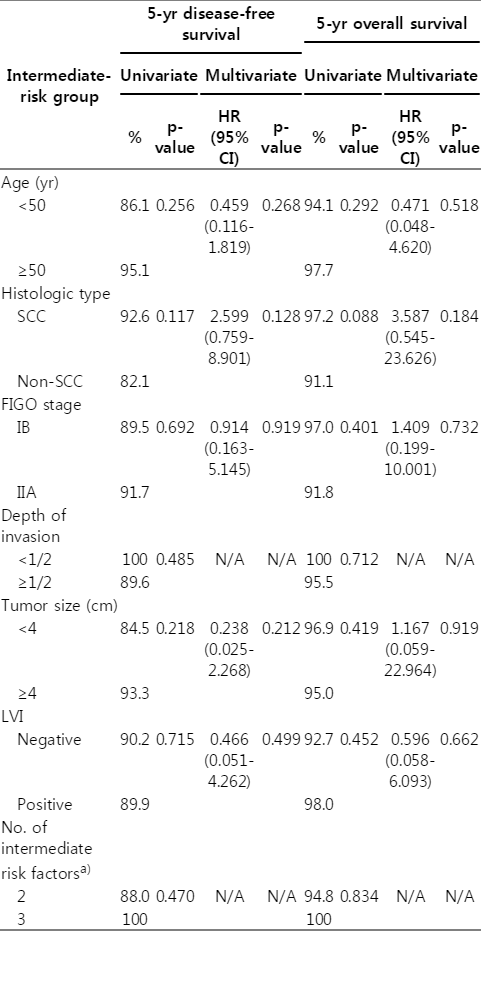
We reviewed 247 patients—149 in the high-risk group and 98 in intermediate-risk group. The median follow-up was 62 months. Loco-regional failure (LRF) alone occurred in 7 patients (2.8%), distant metastasis alone in 37 patients (15.0%) and LRF with DM in 4 patients (1.6%). The 5-year disease-free survival (DFS) and overall survival (OS) rates for both groups were 79.7% and 87.6%, respectively. In the high-risk group, the 5-year DFS and OS probabilities were 72.5% and 81.9%, respectively. Histologic type, pathologic tumor size, and the number of pelvic lymph node (PLN) metastasis were significant prognostic factors for DFS and OS. We suggest a scoring system (0–3) using these prognostic factors to predict poor prognosis in high-risk patients. Using this system, patients with higher scores have higher recurrence and lower survival rates.
Conclusion
In the high-risk cervical-cancer group who received primary surgery and adjuvant CCRT, non-squamous type, large tumor size and the number of PLN metastasis were significant prognostic factors, and the number of these factors was associated with survival rates.
Introduction
Uterine cervical cancer is the fourth most common female cancer worldwide [1] and the sixth most frequently diagnosed cancer for women in Korea [2]. Incidence and mortality associated with cervical cancer have been decreasing continuously for several years, but the survival rate has not improved, despite advancements in diagnosis and treatment.
For staging and determining the best treatment approach for cervical cancer, most oncologists use the International Federation of Gynecology and Obstetrics (FIGO) clinical staging system [3]. Based on the FIGO staging system, a radical hysterectomy or definitive radiotherapy (RT) with or without chemotherapy may be recommended for patients with early stage (IB-IIA) cervical cancer. After primary radical surgery, adjuvant treatment is frequently indicated for early-stage patients who have poor prognostic risk factors to reduce the likelihood of recurrence and improve survival. Several studies have identified high-risk factors for recurrence and mortality, including parametrial invasion (PMI), pelvic lymph node (PLN) metastasis, and a positive resection margin (RM). For patients with any of these risk factors, adjuvant concurrent chemo-radiation therapy (CCRT) is often recommended [4]. Also, RT alone has been recommended as an adjuvant treatment for patients with intermediate risk factors, including lymphovascular invasion (LVI), deep stromal invasion, and large tumor size [5,6].
The aim of this study was to analyze clinical outcomes from cervical cancer and stratify patients into risk groups for prognostic factors for early-stage disease.
Materials and Methods
We retrospectively reviewed FIGO stage IB or IIA cervical cancer patients treated with adjuvant RT or CCRT following primary surgery at Samsung Medical Center between 2001 and 2011. We excluded patients who: received neoadjuvant chemotherapy, did not undergo radical surgery, received adjuvant sequential chemo-radiation therapy, received <45 Gy of adjuvant RT or CCRT, or had small-cell carcinoma with surgery-confirmed metastasis to other organs or to at least one para-aortic lymph node (PALN). All patients were treated with a primary radical hysterectomy and pelvic lymphadenectomy, a removal of all lymph nodes from the external, internal, and common iliac vessels and the obturator fossa nodes, and/or PALN sampling. Adjuvant RT was added if there were positive pathologic results for two or more of the following intermediate-risk factors: LVI, invasion depth of at least half or tumor size ≥4 cm (intermediate-risk group). Adjuvant CCRT was performed if the patient was positive for one or more of the following high-risk factors: PMI, PLN metastasis, or positive RM (high-risk group).
Adjuvant RT was started within 4–6 weeks after surgery. External beam radiotherapy (EBRT) was administered to the entire pelvic region using a conventional four-field box technique. Radiation doses ranged from 45–50.4 Gy at 25–28 fractions and 5 fractions per week. Intracavitary brachytherapy was added after EBRT in patients with positive vaginal RMs and was delivered 3 times a week for a total of 18–24 Gy in 6 fractions. Concurrent chemotherapy regimens consisted of weekly cisplatin for 6 cycles or 5-fluorouracil and cisplatin (FP) every 3 weeks for 2–3 cycles.
Follow-up treatment consisted of a physical examination, pap smear, and squamous-cell carcinoma antigen monitoring every 3 months after completing treatment for the first 2 years, and twice annually for the next 3 years. Imaging, including computed tomography, was performed once or twice every year. The follow-up period began after a radical hysterectomy was performed as the primary treatment. In case of recurrence, we classified failures by recurrent site as follows: local failure (LF) is defined as recurrence at vaginal stump, regional failure (RF) is PLN area or pelvic side wall recurrence without visceral organ metastasis, and distant metastasis (DM) is non-pelvic lymph node including PALN and visceral organ metastasis. Loco-regional failure (LRF) includes LF and RF.
For statistical analyses, we used Mann-Whitney U test and Fisher’s exact test for comparison of characteristics between CCRT and RT groups. Univariate and multivariate analyses were performed with base-line variables, high and intermediate risk factors, and the number of risk factors in each group. Kaplan-Meier survival curves to estimate disease-free survival (DFS) and overall survival (OS), and Cox proportional hazards regression models for multivariate analyses of prognostic factors, and we stratified high-risk group of patient by the number of these prognostic factors. A p-values of multiple tests were corrected using Bonferroni’s method. A p-value < 0.05 was considered significant. We used IBM SPSS ver. 21 (IBM, Armonk, NY, USA).
Results
1. Patient characteristics
Patient characteristics are presented in Table 1. A total of 247 patients were reviewed. The median follow-up period was 62 months (range, 2 to 176 months) and the median age was 48 years (range, 23 to 75 years). Histology revealed that 75.3% of the cases were squamous-cell carcinomas (SCC) and 84.6% and 15.4% of cases were classified as FIGO stages IB and IIA, respectively.
Patients were divided into a high-risk group (n = 149) and an intermediate-risk group (n = 98). In the high-risk group, SCC accounted for 74.5% of cases and 84.5% were FIGO stage IB, while 15.5% were IIA. Presence of high-risk factors based on surgical pathology included positive PMI (31.5%), PLN metastasis (84.6%), and positive RM (6.7%). In 126 patients with PLN metastasis, 2 or more positive PLN were 84 (56.4%). All patients in the high-risk group had one or more high-risk factors. Twenty-six patients (17.4%) had two high risk factors and 4 patients (2.7%) had all three factors.
The proportions of histologic cell types and clinical stages were similar between the intermediate-risk group and the high-risk group. Presence of intermediate-risk factors based on surgical pathology included stromal invasion depth ≥1/2 (96.9%), tumor size ≥4 cm (63.3%), and positive LVI (56.1%). There were 82 patients (83.7%) with two intermediate risk factors and 16 (16.3%) with all factors.
2. Patterns of failure
During the follow-up periods, 48 patients (19.4%) experienced recurrence and Table 2 shows the patterns of failure for both risk groups. In details, distribution of recurrence was as follows: LF 2, RF 4, LF and RF 1, DM without LRF 37, and DM with LRF 4 patients. The most common site of DM was lung (16 patients), and no patients with lung metastasis had loco-regional failure. The second common DM was in PALN (13 patients), and there were 7 isolated PALN metastasis. The second common visceral DM was colorectal metastasis (6 patients).
In the high-risk group, recurrence occurred in 37 patients. Isolated LRF occurred in 7 patients (LF 2 RF 4, and LF and RF 1); DM without LRF occurred in 28 patients; and both LRF and DM occurred in 2 patients. In patients with DM, lung metastasis was the most common site of DM (11 patients), and PALN metastasis was the second incidence of DM (10 patients), and 6 were isolated without other metastasis. Supraclavicular lymph node (SCL) metastasis was in 6 patients and 4 were isolated. Other sites of DM were in colorectum (3 patients) and pelvic bone (2 patients).
In the intermediate-risk group, recurrence occurred in 11 patients. DM without LRF occurred in 9 patients and both LRF and DM occurred in 2 patients, while isolated LRF did not occur in any of our study patients. Distribution of DM was similar with CCRT group; lung 5 patients, PALN 3 patients, and colorectum 3 patients.
3. Survival outcomes and prognostic factors
Our survival analyses of all patients found that the 5-year DFS probability was 79.7% and the 5-year overall survival (OS) probability was 87.6%.
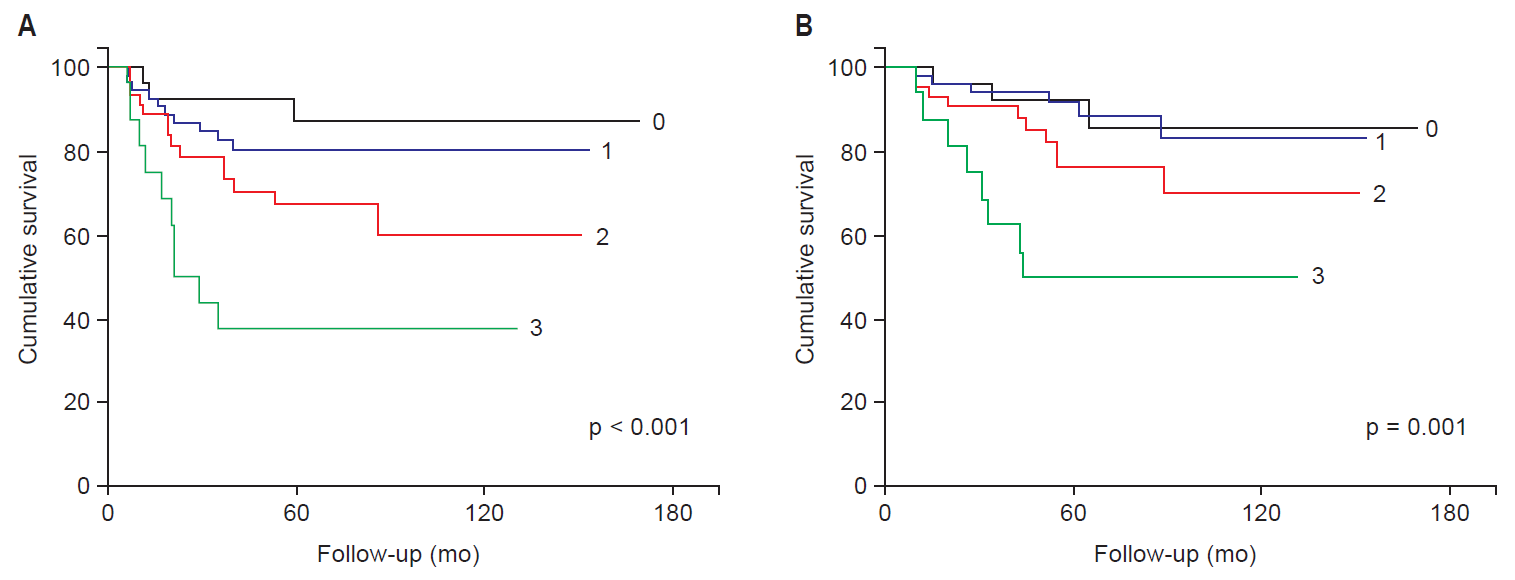
After separating patients into high- and intermediate-risk groups, the 5-year DFS probabilities were 72.5% and 90.0% (p = 0.004), respectively, and the 5-year OS probabilities were 81.9% and 95.7% (p = 0.002) (Fig. 1). According to univariate and multivariate analyses, histologic type, tumor size, and the number of positive PLN were associated with DFS or OS in the high-risk group (Table 3). Non-SCC type was a significant prognostic factor for predicting DFS and OS in multivariate analyses (p = 0.047 and p = 0.037, respectively). Large tumor size was a significant factor for predicting OS in multivariate analyses (p = 0.047), but not for DFS (p = 0.059). Well-known risk factors, including PMI, PLN metastasis, and positive RM, were not significant for any of our survival analyses. The number of positive PLN, however, had a trend of difference with DFS (p = 0.072). When we considered the number of high risk factors including PMI, PLN metastasis and positive RM, we found that patients with two or more positive high risk factors had lower DFS and OS than patients with only one risk factor, although the trend was not significant.
(A) Disease-free survival and (B) overall survival in the high-risk group (HRG) and intermediate-risk group (IRG).
In the intermediate-risk group, none of the factors, including large tumor size and LVI, were associated with DFS and OS in univariate and multivariate analyses (Table 4). We were not able to analyze the association between deep stromal invasion and survival rates in multivariate analyses because none of the patients who did not have deep stromal invasion experienced a recurrence or were censored. Furthermore, we found that the number of intermediate risk factors a patient had was not associated with survival.
4. Prognostic stratification for the high-risk patients
As previously stated, histologic cell type, tumor size and the number of PLN metastasis were prognostic factors for the high-risk group. Thus, we developed a scoring system to identify the patients in the high-risk group who had higher recurrence rate and poorer prognosis (Table 5). Our prognostic risk score incorporates prognostic factors such as non-SCC type (1 point), tumor size ≥4 cm (1 point), and 2 or more PLN metastasis (1 point). Subgroups of patients based on prognostic risk scores had significantly different DFS and OS rates (Fig. 2). The 5-year DFS probabilities for patients with scores of 0–1 and 2–3 were 82.6% and 58.9%, respectively (p = 0.001); OS probabilities were 91.9% and 68.8%, respectively (p = 0.005).
Discussion and Conclusion
We investigated outcomes and prognostic factors for early-stage cervical-cancer patients who received adjuvant therapy following primary surgery. Adjuvant RT is an additional modality to improve local control and overall survival in patients with a poor prognosis based on surgical pathologic findings [4-7]. In our study, the loco-regional control rate was over 90% after adjuvant RT or CCRT following primary surgery and DM accounted for the majority of treatment failures. The recurrence rate was 24.8% in the high-risk group and 11.2% in the intermediate-risk group.
We analyzed each group of patients to determine the relationships between risk factors and survival outcomes. In the intermediate-risk group, we did not find any significant relationships between risk factors and survival outcomes. In the high-risk group, however, histologic cell type, pathologic tumor size, and the number of PLN metastasis were prognostic factors associated with DFS or OS, while other high-risk factors, such as PMI, PLN metastasis, and positive RM, were not associated with survival differences. With histologic cell type, some retrospective studies have found that histologic subtype in early-stage cervical cancer impacts survival [7-10]. Adenocarcinoma was associated with a worse prognosis than SCC type in patients with risk factors identified during surgical pathology [11], whereas other studies found no difference in survival between the two histologic types or reports found survival differences between histologic types among stage-II cases, but not among stage-I cases [12-14]. Also, tumor size was found to only affect survival in the high-risk group. Some studies reported that large tumor size was a poor predictor of recurrence and mortality but did have an effect on increases in PLN metastasis [7,15]. Recent guidelines recommend a definitive CCRT for patients with clinically large tumors (stage IB2 or IIA2). For early-stage patients treated with primary surgery, adjuvant RT alone can be added if two or more positive risk factors are present, including a pathologically large tumor, while patients identified as having any high-risk factors by surgical pathology are recommended to undergo CCRT regardless of tumor size. In this study, however, we found that tumor size affected recurrence and survival, even in the high-risk group. Lymph node metastasis is known as a risk factor of cervical cancer [4], although it does not modify the FIGO clinical stage. In this study, however, PLN metastasis was not significant with survival. Instead of lymph node status, the number of positive PLN metastasis was associated with DFS in univariate analyses. The effect of the number of PLN metastasis has not to be determined, but previous studies reported that there was no difference between survivals of patients with 0 and those with 1 positive node [16-18] and the number of positive lymph nodes might be considerable a prognostic factor in stage IB-IIB cervical cancer patients [19]. These prognostic factors we found in this study should be considered as an additional or secondary risk factor, although these need to be definitively validated in a follow-up study.
Stratification by number of present intermediate-risk factors has been used to determine the likelihood of receiving adjuvant RT [5]; however, few studies have explored the same relationships with high-risk factors. Previous studies of risk factors and treatments did not discuss whether the number of risk factors was an important indicator [4]. We found that an increase in the number of high-risk factors was associated with an increase in likelihood of recurrence and a decrease in survival, although these trends were not statistically significant. Patients in this study were not distributed evenly within their risk groups. Among patients in the high-risk group, 84.6% had PLN metastasis, while a much smaller proportion had recurrent cases with positive RM than other groups with PLN metastasis and PMI. Thus, further evaluations from a well-designed study and a large sample size are needed to assess whether risk stratification by number of risk factors reveals important prognostic factors for high-risk patients.
We focused our risk stratifications on patients with existing or additional prognostic factors, and observed that patients with non-SCC, large tumors, and 2 or more PLN metastasis had poorer prognosis than other participants in our study. Although poorer prognosis in patients with certain conditions, such as large tumor size, adenocarcinoma, or positive PLN metastasis after primary surgery, have been reported [4,7], there are no confirmed criteria for determining which patients need additional treatment; nor is there enough evidence to alter recent treatment guidelines for high-risk patients. Nevertheless, previous studies of patents with locally advanced cervical cancer, from our center and others, have consistently reported that consolidation chemotherapy after primary CCRT might enhance local control and eradicate distant micrometastases [20-23]. A phase-II study that enrolled high-risk early-stage cervical cancer patients as well as locally advanced patients concluded that consolidation chemotherapy had low efficacy [24]. Meanwhile, a recent phase-II study suggested that pelvic CCRT with intensity-modulated radiotherapy followed by consolidation chemotherapy might be highly effective in surgically treated cervical-cancer patients with positive PLN; 3-year progression-free survival and OS among these patients were 88.5% and 93.8%, respectively, and these outcomes are superior to those from historical control groups who received adjuvant CCRT without consolidation [25]. However, there has been no consensus on regimens for consolidation chemotherapy or even adjuvant CCRT. Most patients in our study and in historical control groups were treated with platinum-based chemotherapy, while other highly cited studies used carboplatin plus paclitaxel for CCRT or consolidation chemotherapy. Furthermore, long-term chemotherapy might cause more severe or higher frequency toxicity [24,25]. Also, there have been no randomized trials evaluating consolidation chemotherapy following adjuvant CCRT.
Recently, some randomized trials for more aggressive adjuvant treatment to improve clinical outcomes are ongoing: RTOG 0724, one of phase-III trials considering adjuvant CCRT with or without additional chemotherapy in high-risk patients [26], and GOG 0263, a phase-III trial of adjuvant RT with or without concurrent chemotherapy in intermediate-risk patients [27]. We expect that worthy findings from them will be helpful to decide optimized postoperative treatment plan for cervical cancer patients.
This retrospective study had several limitations. We included cervical-cancer patients treated with adjuvant treatment following a primary radical surgery; thus, a selection bias was present. Also, there was not a uniform protocol for adjuvant chemotherapy regimens in our study. Finally, some of our patient subgroups were very small, which limited our ability to statistically evaluate the effect of the number of risk factors and to determine the significance of additional prognostic factors.
In conclusion, in this study, non-SCC type, large tumor size, and 2 or more PLN metastasis were significant prognostic factors among high-risk patients with early-stage cervical cancer who were treated with radical surgery and adjuvant CCRT. Also, we suspect that the number of these prognostic factors is associated with recurrence and survival.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.