Tumor hypoxia and reoxygenation: the yin and yang for radiotherapy
Article information
Abstract
Tumor hypoxia, a common feature occurring in nearly all human solid tumors is a major contributing factor for failures of anticancer therapies. Because ionizing radiation depends heavily on the presence of molecular oxygen to produce cytotoxic effect, the negative impact of tumor hypoxia had long been recognized. In this review, we will highlight some of the past attempts to overcome tumor hypoxia including hypoxic radiosensitizers and hypoxia-selective cytotoxin. Although they were (still are) a very clever idea, they lacked clinical efficacy largely because of ‘reoxygenation’ phenomenon occurring in the conventional low dose hyperfractionation radiotherapy prevented proper activation of these compounds. Recent meta-analysis and imaging studies do however indicate that there may be a significant clinical benefit in lowering the locoregional failures by using these compounds. Latest technological advancement in radiotherapy has allowed to deliver high doses of radiation conformally to the tumor volume. Although this technology has brought superb clinical responses for many types of cancer, recent modeling studies have predicted that tumor hypoxia is even more serious because ‘reoxygenation’ is low thereby leaving a large portion of hypoxic tumor cells behind. Wouldn’t it be then reasonable to combine hypoxic radiosensitizers and/or hypoxia-selective cytotoxin with the latest radiotherapy? We will provide some preclinical and clinical evidence to support this idea hoping to revamp an enthusiasm for hypoxic radiosensitizers or hypoxia-selective cytotoxins as an adjunct therapy for radiotherapy.
Introduction
Tumor hypoxia is a common feature in many, if not all, human and animal solid tumors, which can occur by two mechanisms: diffusion-limited chronic hypoxia, proposed by Thomlinson and Gray [1] more than 50 years ago where they observed uniform bands of necrotic cancer cells approximately 100–150 μm distant away from blood vessels in human tumors and perfusion-limited acute hypoxia proposed by Brown [2] in 1970’s, which is caused by temporary obstruction or variable blood flow in tumor vessels (Fig. 1). By utilizing the gold-standard Eppendorf probe [3], the partial pressure for oxygen has been shown to be much lower in many types of solid tumors compared to that of the adjacent normal tissues [4]. It is important to note that there is no linear relationship between tumor size and the extent of hypoxia. A preclinical study in human colorectal adenocarcinoma xenografts has reported that small tumors of less than 1 mm in diameter are already being extensively hypoxic and poorly perfused [5].
It has been extensively demonstrated that tumor hypoxia is a major contributing factor for the failure of many anticancer therapies such as surgery [6], chemotherapy [7], and radiotherapy [4]. Tumor hypoxia may also interfere with the efficacy of the latest anticancer immunotherapy. Many functions including differentiation, maturation, and proliferation of various populations of immune cells are known to be negatively affected by hypoxic conditions [8]. Furthermore, it is recently reported that the expression of programmed death-ligand 1 (PD-L1), the major inhibitory ligand towards cytotoxic T cells by binding to its cognate receptor PD-1 [9] is regulated by hypoxia-inducible factor-1 (HIF-1) [10], a master transcription factor well known to be stabilized in hypoxic cancer cells activating various pathways including angiogenesis, glycolytic metabolism, and metastasis [11].
Hypoxia is a direct and negative impact factor for radiotherapy because the molecular oxygen O2 is absolutely necessary to chemically fix DNA free radicals produced by ionizing radiation [4]. In the absence of O2, DNA radicals are repaired by abstracting hydrogen from sulfhydryl (SH) group present in protein [4]. It has been reported that three times higher ionizing radiation dose is required to kill hypoxic cancer cells compared to well-oxygenated cells in order to achieve the equivalent level of cell kill [4]. Because of this negative effect exerted by hypoxia on radiotherapy outcome, much attempts had previously been made to overcome tumor hypoxia. In this review, we will highlight some of such attempts and discuss future perspectives of tumor hypoxia for radiotherapy.
Past Attempts with Hypoxic Radiosensitizers
One of the earliest attempts to overcome radioresistance mediated by hypoxic tumor cells was to increase oxygen levels in the blood stream by making patients breathe 100% oxygen at 3 atmosphere pressure [12]. However, clinical results were mixed and the reason was suggested because this strategy was ineffective in controlling acutely hypoxic cells [13]. In some cases, the use of carbogen (95% O2/5% CO2) has demonstrated a greater benefit than 100% oxygen in increasing oxygen levels in the blood because of vasodilation effect exerted by CO2 [14]. Other approaches were the use of nicotinamide, an agent to increase tumor blood flow [15], artificial blood substitutes carrying increased levels of oxygen such as perfluorocarbons [16], and hyperthermia [17] although none of these treatments were proven to be clinically effective.
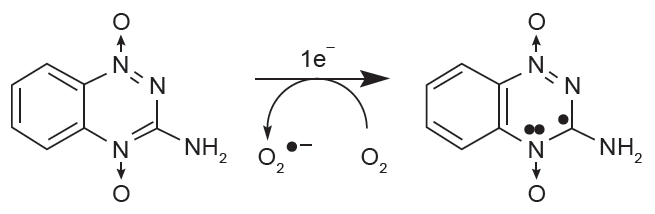
In 1960’s Adams and Cooke [18 ] proposed a clever idea that electron-affinic chemical drugs may act like O2, a potent radiosensitizer. Radiosensitizer would accept thereby react with an electron (Fig. 2A) such as DNA radical produced by ionizing radiation. Radiosensitizers would exert their hypoxic selective mode of action because the chemical reduction would not occur in the presence of O2. O2 either strongly back-oxidizes this reductive chemical reaction (Fig. 2A) or reacts with DNA radical faster than radiosensitizers would. However, unlike O2, these agents are not being metabolized by cells through which they penetrate thus able to diffuse beyond the oxygen diffusion distance [19]. With misonidazole and metronidazole being the prototype members of nitroimidazole class of hypoxic radiosensitizers (other classes of radiosensitizers are reviewed elsewhere by Ahn and Brown [20]) (Fig. 2B), many clinical studies were conducted but revealed rather disappointing results. They had very little efficacy in improving the clinical response while there was a high incidence of peripheral neuropathy when multiple doses were given [21]. Why multiple doses? Repeated administration of radiosensitizers was required because it needed to be given immediately prior to each fraction of 2 Gy, 5 fractions per week for 4–8 weeks of the conventional ‘fractionated’ schedule of radiotherapy. Therefore attempts to increase hydrophilicity thereby lowering peripheral neuropathy of misonidazole had been made and this lead to the development of analogs including etanidazole [22], pimonidazole [23], and nimorazole [19]. Although etanidazole was shown to be significantly less toxic in mice [22] as with misonidazole it caused peripheral neuropathy upon chronic administration [24] and it lacked a significant clinical efficacy in a randomized phase III trial in head and neck cancer patients treated with radiotherapy [25]. Pimonidazole demonstrated approximately 10-fold increase in radiosensitization potency in vitro compared to misonidazole [26] although its dose limiting toxicity was still associated with some immediate effects involving the central nervous system [23]. As with other radiosensitizers, pimonidazole was not effective as an adjunct to radiotherapy and one of the reasons had been suggested to be due to an incomplete randomization of patients between control and test arms such that there were unexpectedly good results obtained in the control arm of the trial [27]. Despite all these disappointing clinical results above, a recent meta-analysis performed in 4,805 head and neck cancer patients in 32 randomized clinical trials revealed that hypoxic modifications such as oxygen breathing, the use of nicotinamide or nitroimidazoles (misonidazole, metronidazole, and etanidazole) offered a significant clinical benefit when loco-regional control and overall survival were used as the endpoint [28]. With the exhausted enthusiasm for hypoxic radiosensitizers, misonidazole is currently being utilized in the clinic as a positron-emission tomography (PET) [29] probe detecting tumor hypoxia [30] and pimonidazole, also known as ‘hypoxyprobe’ is being used to detect the tissue hypoxia in the pre-clinical setting [31]. Doranidazole is the latest member of this class of hypoxic radiosensitizers and has recently shown a significant improvement in the long-term survival of unresectable pancreatic cancer patients when given with 25 Gy postoperative radiotherapy [32].
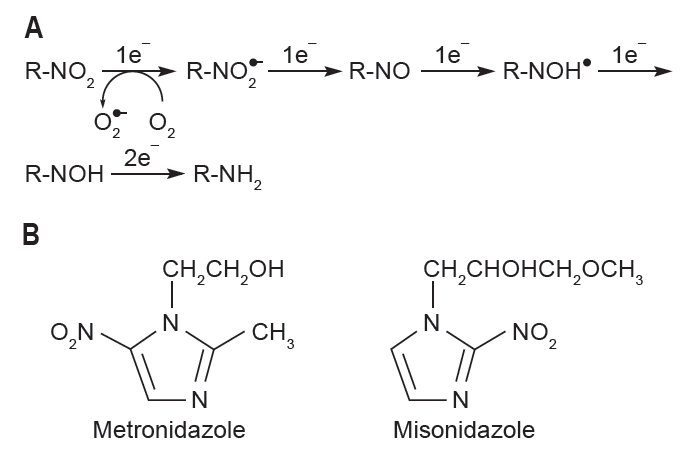
Hypoxic radiosensitizers. (A) Hypoxic selective mechanism of action for nitroimidazole class (R-NO2) of radiosensitizers. (B) Chemical structure of metronidazole and misonidazole.
In the mid 80’s, Brown [33] developed a novel agent named tirapazamine that can selectively kill hypoxic cells thereby turning tumor hypoxia from a problem to a selective treatment advantage (Fig. 3). This so-called ‘hypoxia-selective cytotoxin’ tirapazamine demonstrated hypoxic cytotoxicity ratio of 50–200 in murine and human cancer cell lines although in vivo hypoxic cytotoxicity was somewhat lower than that in vitro [34]. Tirapazamine had been extensively tested in combination with radiation or cisplatin in many preclinical and clinical studies [34]. With the dose-limiting toxicities of reversible muscle cramping, nausea, and vomiting, many phase I and II trials demonstrated promising results in patients particularly with the head and neck or the lung cancers [34]. However, despite promising earlier clinical results, most of the phase III clinical trials results turned out that tirapazamine did not add a significant improvement in prolonging the overall survival for patients treated with chemotherapy [34] or chemoradiation [29], likely to be due to the lack of information on the extent of patients’ tumor hypoxia upon patient selection and randomization of the trial.
Hypoxia Imaging–Important Lessons We Have Recently Learned from 18F-MISO and Tirapazamine
Although misonidazole lacked clinical efficacy as a radiosensitizer, 18F-misonidazole (18F-MISO) is currently being utilized in the clinic as a PET probe imaging tumor hypoxia [35]. With recent 18F-MISO PET imaging studies, we have acquired some very valuable information.
First, Trans-Tasman Radiation Therapy Oncology Group (RTOG) 98.02 study in advanced squamous cell carcinoma of the head and neck patients has demonstrated that tirapazamine, which had otherwise failed in phase III clinical trials above [29] could be dramatically effective in lowering the locoregional failure if patients were selected based on tumor hypoxia by 18F-MISO PET scans in prior [36]. In that study, only one of 19 head and neck cancer patients had recurrence when tirapazamine was given in combination with chemoradiation whereas 8 out of 13 patients recurred if they received 5-fluorouracil in place of tirapazamine [36] (Fig. 4). These results thus indicate that all those clever ideas developed in 1970’s and 1980’s would work well if we could better identify patients with significant hypoxic fractions being present in their tumors.

Locoregional failure by treatment arm and hypoxia. Tirapazamine offers a significant benefit lowering the locoregional failures in head and neck cancer patients with hypoxia (red box). TPZ/CIS, tirapazamine/cisplatin; RT, radiotherapy. Modified from Rischin et al. [36] with permission of the American Society of Clinical Oncology.
Second, 18F-MISO PET imaging has shown that tumor hypoxia can dramatically change over time, as early as a few hours up to days [37]. Although there had been some attempts to measure the dynamics and kinetics of changes in tumor hypoxia, it was largely concluded that it occurred rather subtly and unpredictably, and that it frequently associated with immature blood vessels [37]. Due to the unpredictable nature, changes in tumor hypoxia can significantly complicate the latest radiotherapy technology, for example intensity-modulated radiotherapy (IMRT; see below ‘Recent advances in radiotherapy technology’) dose-painting technology where additional boost dose of irradiation can be given to hypoxic regions of tumors. For example, Lin et al. [38] have reported that some patients whose tumor hypoxia varies significantly from day to day would not benefit such dose-painting radiotherapy because ‘newly hypoxic’ regions of tumors would receive the dose that is not high enough to kill hypoxia while ‘now no-longer’ hypoxic regions would be treated with unnecessarily high dose of irradiation (Fig. 5).
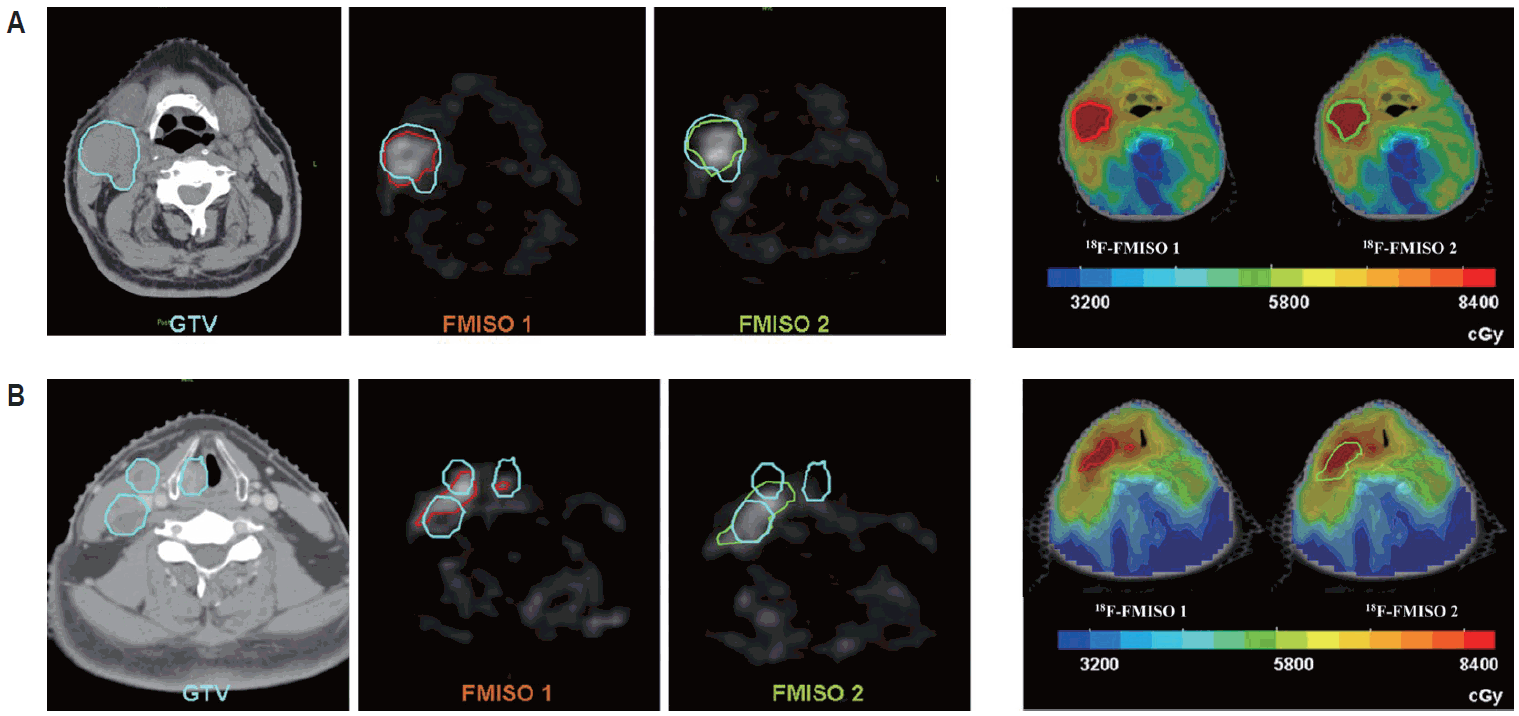
Tumor hypoxia is dynamically changing. (A) A positron emission tomography-computed tomography (PET-CT) image (left) and intensity-modulated radiotherapy (IMRT) treatment (right) for a patient whom do not demonstrate a significant change in the tumor hypoxia as examined by two consecutive 18F-misonidazole (18F-MISO) images (left). (B) A significant change in the location of tumor hypoxia is noted in this patient between 1st (red area) and 2nd (green area) images of F-MISO PET (left). Upon IMRT treatment (right), newly hypoxic areas (green) would receive the dose not enough to kill hypoxic cells while those tumor areas that are no longer hypoxic would experience overdose of irradiation. Adapted from Lin et al. [38] with permission of Elsevier.
Recent Advances in Radiotherapy Technologies
The ways to deliver radiotherapy have been drastically changed over the last decades. Previously, conventional radiotherapy had been consisted of relatively small doses (1.2–3 Gy/fraction) daily fraction over weeks or months based on a small survival advantage of the normal tissues over the tumors [39]. Importantly, this treatment regimen has led to ‘reoxygenation’ phenomenon, in which surviving hypoxic tumor cells at a given dose become oxygenated by the time of the next fractionation treatment [40], which has turned out to be one way to effectively kill hypoxic tumor cells. Much of radiobiological work with this fractionated irradiation has taught us that the normal tissue toxicity occurring by fractionated irradiation has been shown to be dependent upon several factors including the fraction size, tissue type, and total doses of radiation [39]. Based on this understanding, some of altered fractionation schemes were further developed: hyperfractionation of smaller fraction doses, increased numbers of fractions, and interfractionation intervals by at least 6 hours (because sublethal damage repair has shown to occur within 6 hours); or accelerated fractionation where the overall treatment time being shortened in order to reduce accelerated repopulation of tumor cells [39]. These modifications in fractionated irradiation have shown to improve the local control rates while the late normal tissue toxicity not being significantly different [41].
Recent technological advancement has allowed us to deliver single large fraction of 15–20 Gy precisely to the tumor-bearing volume, known as stereotactic radiosurgery (SRS) for cranial lesions and stereotactic body radiotherapy (SBRT) for other extracranial sites [39]. Use of such technologies has significantly improved the clinical response: for example recent results from RTOG 0236 protocol in non-operable early-stage lung cancer patients treated with 3 fractions of 20 Gy/fraction SBRT have shown 3 year local control and survival to be 98% and 56%, respectively, the results being comparable to surgery [42]. Computer-controlled beam shapes with multileaf collimators and intensity modification by using a wedge-shaped compensating filter have led to IMRT allowing highly conformal distributions of high doses of radiation in target volumes while maintaining the low levels of radiation to the surrounding normal tissues [39].
Tumor Hypoxia with SBRT—Back to Square One?
Without a doubt, the recent advancement in radiotherapy technology has brought superior clinical responses in numerous cancer patients. However has this technology overcome the problem of tumor hypoxia? A recent modeling study by Carlson et al. [43] suggested that assuming daily fractionation and full reoxygenation between fractions, tumor hypoxia is actually more serious problem with SBRT. Tumor cell survival can increased up to 100-fold as we choose ‘less number’ of fractions and ‘at higher dose’ per fraction (‘A’ in Fig. 6), which may be equivalent to current SBRT. In addition, an increase in hypoxic fraction from 10% to 30% has modeled to reflect nearly 10 times further resistance of tumor cells at this high dose hypofractionated regimen [43] (‘B’ in Fig. 6). The reason has been suggested largely to be due to a reduction in interfractionation reoxygenation associated with this single high dose SBRT.
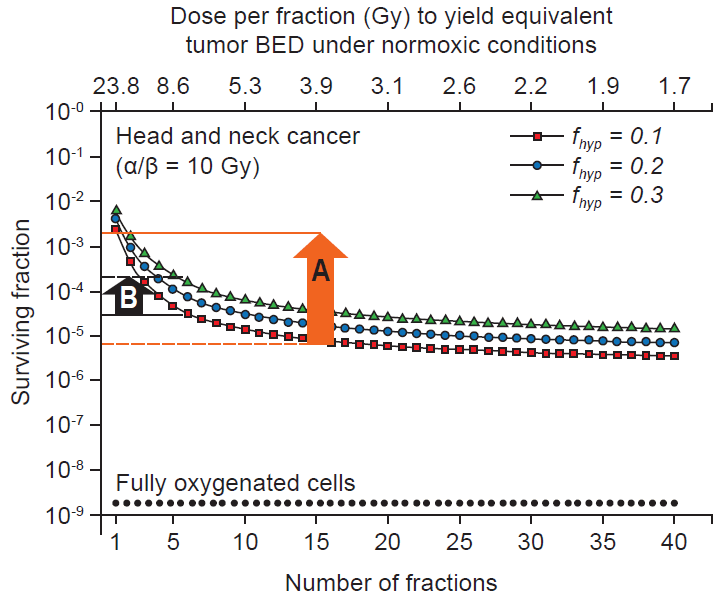
Total surviving clones as a function of dose per fraction assuming daily fractionation and full reoxygenation between fractions. A: For tumors with 10% hypoxia (fhyp = 0.1; red line) there is more than 2 logs (100-fold) of cell survival advantage (pointed with the orange arrow) as we reduce the number of fractionations (as we shift right to left). B: As hypoxic fraction increases from 10% (fhyp = 0.1; red line) to 30% (fhyp = 0.3; green line), there is additional 1 log (10-fold) of cell survival increase (pointed with the black arrow) at 5 fractionation treatment, at which the cell survival is already being much higher than that by hyperfractionation. Adapted from Carlson et al. [43] with permission of Elsevier.
What can we do about it? An obvious way would be to deliver the next fraction of SBRT to tumors where the tumor reoxygenation would be at its maximum. This may even mean ‘personalized’ radiotherapy based on monitoring individuals’ changes in tumor hypoxia, which in reality would be extremely difficult to do so. In fact, one of current dilemma for SBRT is that there are no standardized intervals between each hypofractionation. For examples, Timmerman et al. [44] prescribed 18 Gy per fraction × 3 fractions within 2 weeks, while Nagata et al. [45] prescribed 12 Gy per fraction × 4 over 5–13 days. In one study 50 Gy was delivered over 4 consecutive days, meaning 12.5 Gy every day for 4 days [46]. Surprisingly all those studies demonstrated good clinical outcome despite differences in their fractionation intervals. Does it mean that SBRT does not rely on reoxygenation? Probably the good clinical response may derive from such ‘ablative’ doses of ionizing radiation producing tremendous of DNA damages in the affected areas. We have recently investigated if we can determine changes in tumor hypoxia before and after a single high dose of 20 Gy irradiation by utilizing various techniques including 18F-MISO PET imaging, pimonidazole, and bioluminescence imaging in a mouse tumor model [47]. We found that tumor hypoxia did not significantly change when examined at 6 hours, 2 days, and 5 days postradiation although we interestingly observed that such high dose irradiation caused a rapid but transient vascular collapse [47]. There are similar attempts currently undergoing in lung cancer patients by Kelada et al. [48]. In their preliminary study, there was an increase in tumor hypoxia shortly after 18 Gy measured by 18F-MISO PET imaging although inter- and intra-patient variability was too high to firmly conclude their findings.
If inter- and intra-individual tumor hypoxia changes are too variable to predict, an easy alternative (at least until we have a better technology imaging/monitoring real time changes of tumor hypoxia) would be to use radiosensitizers. As a matter of fact, radiosensitizers of many preclinical studies of in vivo [49,50] and in vitro [51] in earlier days had been tested at much higher radiation doses (5–30 Gy), equivalent to today’s SBRT and demonstrated that it would work much better with high doses of irradiation [51] (Fig. 7). A recent preclinical study by Wittenborn and Horsman [52] has demonstrated the proof-of-concept that TCD50 (radiation dose to cure 50% of tumors) can be decreased from 29.7 Gy to 2.5 Gy when 3 × 15 Gy was combined with nimorazole 30 minutes prior to each fraction (Table 1). Clinical enthusiasm may have been exhausted and doomed because of disappointing clinical results with nearly all of hypoxic radiosensitizers tested (see above ‘Past attempts with hypoxic radiosensitizers’). However, there is a clinical study as early as in 1980’s that 9 out of 10 vulvovaginal cancer patients achieved the local control when metronidazole was given as a radiosensitizer with high-dose radiation [53]. A recent clinical study with doranidazole has demonstrated a significant improvement in 3-year survival in advanced pancreatic cancer patients when it was combined with 25 Gy intraoperative radiotherapy [32]. These preclinical and clinical results thus indicate that hypoxic radiosensitizers or hypoxia-selective cytotoxins may work well with the latest high dose hypofractionation radiotherapy.

Survival curves of Chinese hamster ovary (CHO) cells irradiated under oxic (O2), hypoxic (N2), or hypoxic conditions in the presence of 1.5 mM misonidazole (red line) by 270 kVp X-ray irradiation at (A) high or (B) low dose range. Note that sensitization enhancement ratio (yellow arrows) of misonidazole is larger at higher irradiation dose range (A). Adapted from Palcic et al. [51] with permission of publisher.
Future Perspectives
Because of tumor hypoxia and the normal tissue complication, other radiotherapy modalities such as charged particle beam radiotherapy of proton or carbon ion are being actively developed and utilized to treat cancers world-wide. Compared to X-ray, carbon ion offers advantages of superior cell kill and a potential to decrease normal tissue toxicity [54]. This is first because carbon ion is of high linear energy transfer (LET), it does not depend on the oxygen effect for producing cell kill. Second, Bragg peak, the extent of energy loss of radiotherapy along the penetration depth of tissues as it travels through is even optimal with carbon ion therapy among other heavier ions–carbon ion has the lowest entry relative biological effectiveness (RBE; the ratio in the dose of one type of ionizing radiation to another at the same level of biological effects) while it demonstrates the maximal RBE in Bragg peak [54]. Some of the current limitation of this approach may include a limited availability of the facility, high treatment costs, and rather short clinical history compared to X-ray ionizing radiation.
In an attempt to take an advantage of the existing clinical linear accelerator (LINAC), the newest effort has been suggested to use ultra-high dose rate FLASH irradiation [55]. The concept was first introduced by Favaudon et al. [56] demonstrating that the ultra-high dose rates in the range of 40–60 Gy per second resulted in a significantly reduced lung toxicity while the local control of tumors was similar compared to conventional 17 Gy irradiation. Recently, Schuler et al. [55] are actively tuning and modifying a clinical LINAC instrument for electron FLASH offering an easy set up and more homogenous dose distribution in tumors than photon-induced radiotherapy.
Optical approaches in imaging oxygen tensions and delivering oxygen to hypoxic regions of tumors are also being actively developed in the preclinical settings. Studies have shown that redox ratio of autofluorescence for endogenous molecules such as hemoglobin could reflect the oxygen tensions in tumors [57] and that this can predict the tumor response to irradiation in FaDu head and neck squamous cell carcinoma xenograft models in mice [58]. This approach may be limited to determination of the oxygen partial pressure in blood vessels of tumors not necessarily reflecting those within tumors. In this respect, we have recently developed an optical oxygen sensor consisting of ruthenium (Ru) complex encapsulated in amphiphilic polymer where we can quantify the oxygen concentrations based on the ratiometric analyses of Ru phosphorescence signal in cancer cells [59]. Dewhirst group has also reported a similar approach of boron nanoparticle where they have quantified the oxygen in tumors implanted underneath in vivo dorsal window chamber [60]. A platinum complex of porphyrin complexes has recently demonstrated low yet heterogeneous oxygen tension distributions in the bone marrow of mice by using phosphorescence lifetime imaging [61]. Nanoparticle approaches delivering the molecular oxygen to tumors are also very exciting. Prasad et al. [62] have shown that albumin-coated manganese oxide nanoparticles release the molecular oxygen by H2O2 produced by oxidative stress within the tumor microenvironment, which can increase the tumor oxygenation up to 45% in tumors leading to a significantly increased radiotherapy efficacy in breast cancer models in mice. Another interesting approach using nanoperfluorocarbon of high oxygen dissolving properties, Song et al. [63] have recently demonstrated that these agents can pick up the oxygen from the lung and selectively release it in tumors only by ultrasound stimulation in hyperoxic breathing mice, thereby improving the tumor response to radiotherapy and photodynamic therapy [63]. Future studies including investigation of pharmacokinetics of these agents would be warranted in order to move forward to be applied in the clinical setting.
Conclusions
Small doses of hyperfractionation regimen had implemented to lower the normal tissue toxicity produced by ionizing radiation, which has brought us an important radiobiological concept ‘reoxygenation.’ While it turned out to kill hypoxic tumor cells efficiently, it paradoxically acted as a major disadvantage towards our previous efforts for hypoxic radiosensitizers and hypoxia-selective cytotoxins, the two great ideas which may have worked so well otherwise. The latest technology advancement in radiotherapy using high doses of hypofractionation regimen has totally changed the picture of cancer treatment and brought superb clinical responses. However, recent modeling studies suggest that tumor hypoxia would be a significant problem with the latest radiotherapy because the interfractionation reoxygenation would be minimal. While we do not have reoxygenation phenomenon to interfere with hypoxic radiosensitizers and/or hypoxia-selective cytotoxins, this may be a perfect time to reconsider combining these with the latest radiotherapy technique (Fig. 8).

A diagram demonstrating development of hypoxic radiosensitizers and hypoxia-selective cytotoxins. Reoxygenation by the conventional low doses hyperfractionation radiotherapy had previously been interfered with proper activation of those compounds in tumors. Because interfractionation reoxygenation is now being predicted to be low with the latest high doses hypofractionation radiotherapy stereotactic body radiotherapy (SBRT), perhaps it is sufficiently reasonable to combine hypoxic radiosensitizers or hypoxia-selective cytotoxins with such radiotherapy techniques. PET, positron-emission tomography.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by Ministry of Science, ICT, and Future Planning, Korea (NRF-2015K2A1A2070880 and NRF-2015M2B2A9029247); and Ministry of Education, Korea (BK21 Plus 10Z20130012243). Hong BJ (NRF-2013H1A2A1032808), Kim JW (NRF-2016H1A2A1908006), and Kim YE (NRF-2012H1A2A1002871) are Global PhD fellowship recipients supported by Ministry of Science, ICT, and Future Planning, Korea. Jeong H is a Cheong-Am Science Fellow supported by POSCO Cheong-Am Foundation, Korea.