Dosimetric comparison of IMRT versus 3DCRT for post-mastectomy chest wall irradiation
Article information
Abstract
Purpose
To compare the dose distribution of three-dimensional conformal radiation therapy (3DCRT) with intensity-modulated radiation therapy (IMRT) for post-mastectomy radiotherapy (PMRT) to left chest wall.
Materials and Methods
One hundred and seven patients were randomised for PMRT in 3DCRT group (n = 64) and IMRT group (n = 43). All patients received 50 Gy in 25 fractions. Planning target volume (PTV) parameters—Dnear-max (D2), Dnear-min (D98), Dmean, V95, and V107—homogeneity index (HI), and conformity index (CI) were compared. The mean doses of lung and heart, percentage volume of ipsilateral lung receiving 5 Gy (V5), 20 Gy (V20), and 55 Gy (V55) and that of heart receiving 5 Gy (V5), 25 Gy (V25), and 45 Gy (V45) were extracted from dose-volume histograms and compared.
Results
PTV parameters were comparable between the two groups. CI was significantly improved with IMRT (1.127 vs. 1.254, p < 0.001) but HI was similar (0.094 vs. 0.096, p = 0.83) compared to 3DCRT. IMRT in comparison to 3DCRT significantly reduced the high-dose volumes of lung (V20, 22.09% vs. 30.16%; V55, 5.16% vs. 10.27%; p < 0.001) and heart (V25, 4.59% vs. 9.19%; V45, 1.85% vs. 7.09%; p < 0.001); mean dose of lung and heart (11.39 vs. 14.22 Gy and 4.57 vs. 8.96 Gy, respectively; p < 0.001) but not the low-dose volume (V5 lung, 61.48% vs. 51.05%; V5 heart, 31.02% vs. 23.27%; p < 0.001).
conclusions
For left sided breast cancer, IMRT significantly improves the conformity of plan and reduce the mean dose and high-dose volumes of ipsilateral lung and heart compared to 3DCRT, but 3DCRT is superior in terms of low-dose volume.
Introduction
Breast cancer is the most common cancer in females, both worldwide and in India [1]. In contrast to Western world, most of the patients here present with advanced stage due to lack of mass screening programmes and awareness; so modified radical mastectomy (MRM) is performed more often than breast conservative surgery (BCS). However, with recent increased use of mammography and neoadjuvant therapies, the number of BCS is increasing, but still lacking behind MRM. Most of these patients require post-mastectomy radiotherapy (PMRT) to decrease locoregional recurrence [2-5]. PMRT is recommended in patients with 4 or more positive axillary lymph nodes (ALN) and should be strongly considered in patients with 1–3 positive ALN. In patients with negative nodes, PMRT is indicated for tumours more than 5 cm or positive pathological margins [6].
Intensity-modulated radiation therapy (IMRT) has proved to be superior to three-dimensional conformal radiation therapy (3DCRT) in various sites like head and neck, central nervous system, lung, prostate, etc. IMRT, by virtue of multileaf collimators, modulates fluence and divide a beam into small beamlets to prescribe maximum dose to the target with minimum dose to the critical organs. In case of chest wall irradiation, lung and heart remain two most important vital organs, irradiation of which always causes concern to radiation oncologists. This becomes even more complicated as most of the chemotherapeutic agents used to treat breast carcinoma like anthracyclines, taxanes and trastuzumab, possess cardiotoxic potential. IMRT employs an advanced computer program to precisely map your radiation dosage in three dimensions, based on the breast tumor’s size, shape and location. IMRT directs radiation at the breast tumor and modulates the intensity of the radiation beams with laser accuracy, helping to spare healthy tissue surrounding the breast tumor. IMRT allows each dose of radiation to be custom-tailored according to the exact geometrical shape of the breast tumor [7]. On the other hand, IMRT increases integral dose to normal healthy tissue, increasing concern about second malignancy in long-term survivors.
In breast, most of the studies from Western world have been performed on whole breast radiotherapy after BCS; the data on PMRT is scarce. So the present study was carried out at our department to compare the dosimetry in post-MRM patients of left breast with 3DCRT and IMRT.
Materials and Methods
One hundred and seven previously untreated post-MRM female breast cancer patients older than 18 years with histologically confirmed infiltrating ductal carcinoma of unilateral left breast without evidence of distant metastasis or second malignancy were found eligible during July 2014 to June 2016. All patients were planned for adjuvant radiotherapy to the chest wall with inclusion of mastectomy scar. All patients were immobilized while free breathing using a thermoplastic mould in supine position over a breast board fixed on the couch with both arms extended above their head onto arm rests, abducted and externally rotated. Scar sites, drain sites and breast borders were marked using lead markers. Scout view was obtained to assess patient position. Patients underwent both non-contrast computed tomography (CT) simulation and treatment in this position. The 5-mm CT cuts were taken once optimal patient position was confirmed. Supraclavicular fossa (SCF) was irradiated when there was histopathological evidence of 1 or more axillary node metastases, inadequate lymph node dissection (less than 10 nodes examined pathologically) or when neoadjuvant chemotherapy was administered prior to definitive surgery.
The clinical target volume (CTV) was contoured according to the Radiation Therapy Oncology Group (RTOG) breast cancer atlas guidelines [8]. The Planning Target Volume (PTV) was then generated by giving 1 cm margin to CTV. Organs at risk (OAR) contoured included heart, lung, spinal cord, esophagus and contralateral breast. Lung was contoured in pulmonary window, with inclusion of all inflated and collapsed, fibrotic and emphysematous lung and small vessels extending beyond the hilar region. Hila, trachea, and main bronchus were excluded from the lung volume. The heart was contoured along with the pericardial sac, beginning superiorly at the level of the inferior aspect of the pulmonary artery passing the midline, and extending inferiorly upto the apex of the heart. The pulmonary trunk, root of the ascending aorta, and superior vena cava were excluded.
All patients were planned on Siemens ONCOR Expression linear accelerator machine with 6-MV beam. Sixty-four patients were treated with 3DCRT and 43 with IMRT with dose of 50 Gy in 25 fractions with 2 Gy per fraction, 1 fraction per day for 5 days per week. In 3DCRT, PMRT was delivered through tangential field technique, in which the chest wall, with a small portion of lung, is included in the irradiated volume. The chest wall was treated with medial and lateral opposed tangential beams and nodal basins received either radiotherapy through an anterior field with gantry angled 10°–15° to avoid the spinal cord and esophagus. Attention was given to geometric match of SCF field and chest wall field to avoid junctional overdose. In IMRT, chest wall was irradiated using 4–6 fields creating a butterfly shaped planning. IMRT planning was inverse planned. Gantry angled ranged from 285° to 325° for fields from medial sides and 115° to 155° for fields from lateral sides. The monitor units ranged from 95 to 160 for each beam.
1. Dosimetric analysis
The treatment was planned with a goal of 100% volume of PTV to be covered by 95% isodose line. Dose homogeneity was optimized using wedges and ‘field-in-field’ technique using multi leaf collimators. Data collected included the volume of PTV receiving greater than 95% to 107% of prescribed dose (V95 and V107); the dose delivered to 98% (Dnear-min, D98) and 2% (Dnear-max, D2) of the volume of PTV; and mean dose of the PTV (Dmean) from the dose-volume histogram (DVH). Dose homogeneity index (HI) and conformity index (CI) were calculated according to definition proposed by the International Commission on Radiation Units and Measurements (ICRU) Report 83 [9]. HI was defined as the difference between the near-maximum and near-minimum dose normalised to the median dose,
where D2 and D98 are the dose received by 2% (Dnear-max) and 98% (Dnear-min) volume of PTV; and Dp is the prescribed dose. The ideal value is zero; an HI value approaching zero indicates a more homogenous dose distribution within the PTV and it increases as homogeneity decreases. CI was defined as the ratio of volume of tissue receiving at least 95% of the prescribed dose divided by the volume of the PTV. The closer the CI to one, the more conformal is the plan.
For evaluation of OAR, percentage volume of ipsilateral lung receiving 5 Gy (V5), 20 Gy (V20), and 55 Gy (V55) and the mean lung dose were calculated for lung. Similarly for heart, the percentage volume receiving 5 Gy (V5), 25 Gy (V25), and 45 Gy (V45) and the mean heart dose were extracted from the DVH. The treatment plan was accepted if the volume of heart receiving 25 Gy was ≤10% (i.e., V25 heart ≤10%) and the volume of ipsilateral lung receiving 20 Gy was ≤ 35% (i.e., V20 ipsilateral lung ≤35%).
2. Statistical analysis
For statistical analysis, all data were recorded and analysed on Microsoft Excel 2007 and Statistical Package for Social Sciences (SPSS) version 20.0 (IBM Corp., Armonk, New York, USA). The t-test for two independent means was used for quantitative data. The p-value reports were two tailed and an alpha level of 0.05 was used to assess statistical significance.
Results
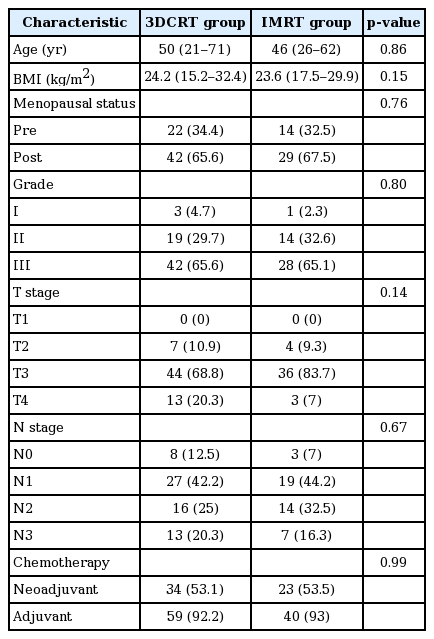
Baseline patient and tumor characteristics are shown in Table 1 and were found to be comparable between the two groups. PTV parameters are shown in Table 2. It can be seen that although CI was significantly improved with IMRT (1.127 vs. 1.254, p < 0.001) compared to 3DCRT, HI was similar (0.094 vs. 0.096, p = 0.83). No significant difference was noted between Dnear-max, Dnear-min, Dmean, V95 and V107 with the two techniques.
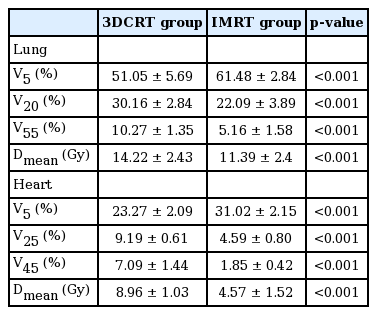
The beam arrangement and DVHs of 3DCRT and IMRT plans are shown in Fig. 1. Dosimetric parameters for lung and heart are shown in Table 3. For lung, IMRT in comparison to 3DCRT significantly reduced the high-dose volumes (V20, 22.09% vs. 30.16%; V55, 5.16% vs. 10.27%; p < 0.001) and the mean dose (11.39 Gy vs. 14.22 Gy, p < 0.001). Similarly for heart also, IMRT in comparison to 3DCRT significantly reduced the high-dose volumes (V25, 4.59% vs. 9.19%; V45, 1.85% vs. 7.09%; p < 0.001) and the mean dose (4.57 Gy vs. 8.96 Gy, p < 0.001). However, 3DCRT proved to be superior to IMRT in terms of low-dose volume for both the lung (V5, 51.05% vs. 61.48%; p < 0.001) and the heart (V5, 23.27% vs. 31.02%; p < 0.001).
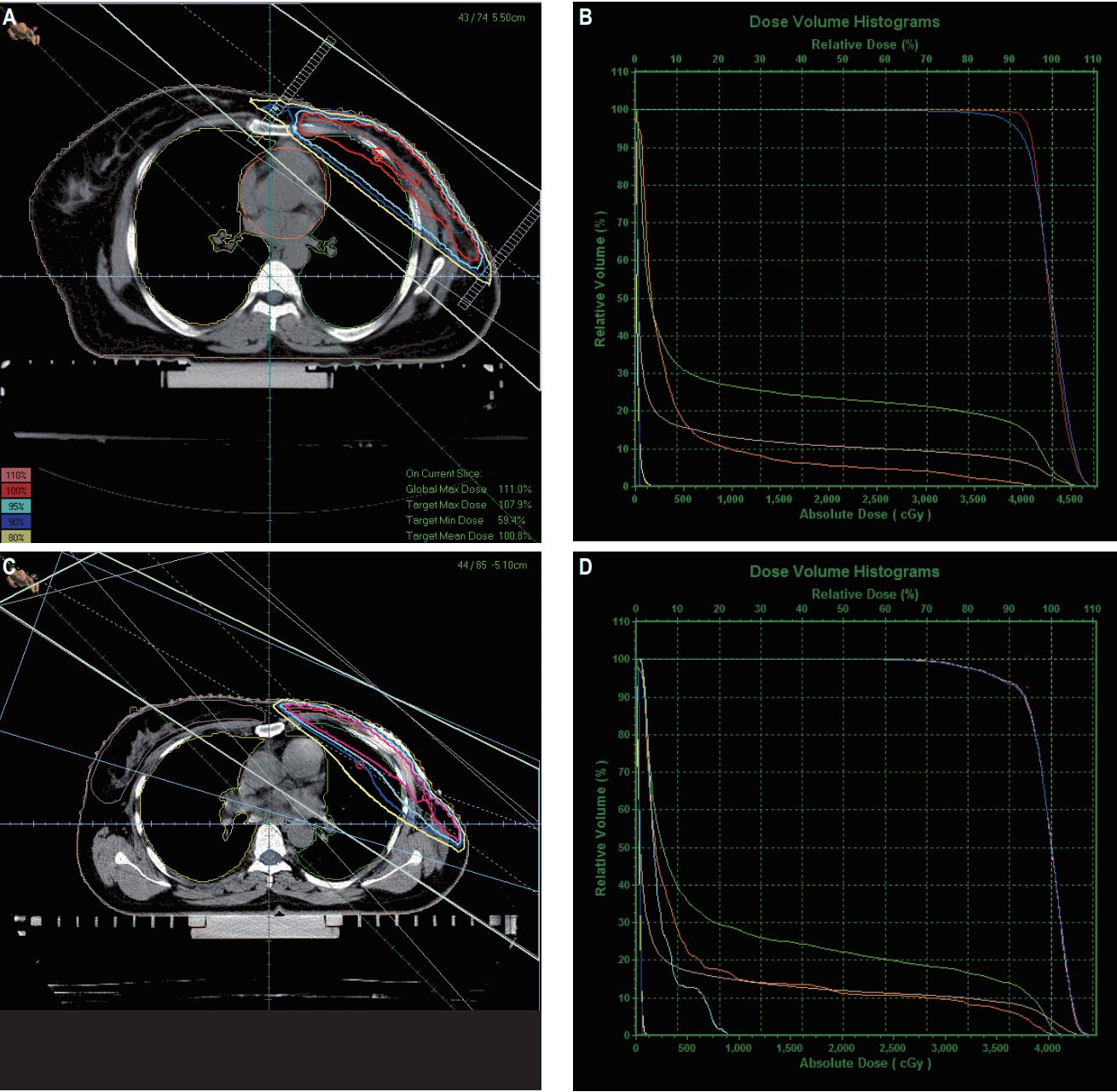
Beam arrangement and dose volume histograms of three-dimensional conformal radiation therapy (A, B) and intensity-modulated radiation therapy (C, D) plans.
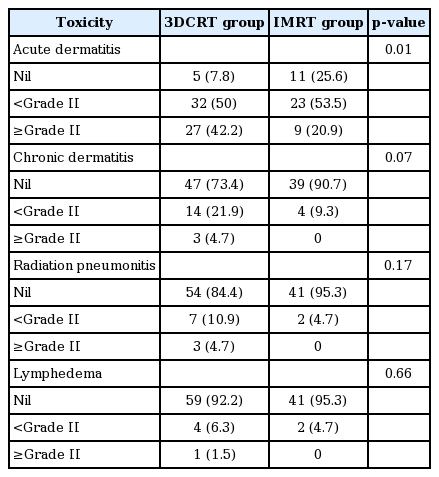
Acute toxicities are shown in Table 4. Acute dermatitis were significantly lower in IMRT group (p = 0.01), but the difference of radiation pneumonitis (p = 0.17) and lymphedema (p = 0.66) between the two groups was not statistically significant.
Discussion and Conclusion
A number of studies have demonstrated dosimetric benefit of IMRT compared to 3DCRT for the whole breast in early breast cancer patients but for post mastectomy chest wall irradiation, such data is scarce. Many studies have reported lower doses to the ipsilateral lung, contralateral lung, contralateral breast, heart, and left anterior descending artery using IMRT technique for whole breast radiotherapy [10]. Fiorentino et al. [11] compared 3DCRT and 4-fields IMRT treatment plans, in terms of target dose coverage, integral dose and dose to organs at risk (OARs) in early breast cancer and concluded 4-fields IMRT technique significantly reduced the dose to OARs and normal tissue, with a better target coverage compared to 3DCRT. Since the anatomy of chest wall is entirely different from that of the whole breast, differences exist between the target volumes of these two. This might have an impact on the resulting dose distribution, both to the PTV and OAR. However, in comparing results of our study on PMRT with earlier studies on BCS, the results are at par except for the cosmetic analysis, which could not be done in case of PMRT.
All our plans had the PTV95% coverage values of >95% of prescription dose. In literature [12,13], various planning studies have shown the PTV95% coverage values ranging from 90% to 97%. Hong et al. have showed that the use of equally spaced gantry angles not only improves HI and CI but also reduces the volume of critical normal tissues [14]. In the present study, statistically significant improvement was noted in CI with IMRT compared to 3DCRT (1.127 vs. 1.254, p < 0.001) [15,16]. However, no significant difference was noted in HI (0.094 vs. 0.096, p = 0.83). Similar results have been reported by Moorthy et al. [15] (CI, 0.14 vs. 0.18, p = 0.01; HI, 1.01 vs. 1.03, p = 0.45) and Rudat et al. [16] (CI, 0.32 vs. 0.25, p = 0.03; HI, 0.73 vs. 0.77, p > 0.05). However, Li et al. [17] concluded that IMRT neither significantly improved CI (1.42 vs. 1.41, p = 0.13) nor HI (0.13 for both groups, p = 1.0); whereas Beckham et al. [18] concluded that IMRT significantly improved not only CI (0.91 vs. 0.48, p < 0.05) but also HI (0.95 vs. 0.74, p < 0.05).
Radiation pneumonitis is one of the most common side effects following PMRT. For patients treated with 3DCRT, the volume of lung receiving 20 Gy i.e., V20 has been found to predict the risk of symptomatic radiation pneumonitis in literature [19-21]. However, there is no absolute safe dose below which there is no pneumonitis. In the present study, both the mean dose and V20 of ipsilateral lung were significantly lower for IMRT compared to 3DCRT (11.39 Gy vs. 14.22 Gy and 22.09% vs. 30.16% respectively; p < 0.001). In a study over 36 post-lumpectomy breast cancer patients, Moorthy et al. [15] concluded that IMRT in comparison to 3DCRT had significantly lower V20 lung (22.4% vs. 37.9%, p = 0.03). In a study over 20 unselected patients, Rudat et al. [16] concluded that tangential beam IMRT significantly reduced the ipsilateral mean lung dose by an average of 21% (11.29 Gy vs. 14.37 Gy, p < 0.01) and D30 by 43% (9.60 Gy vs. 16.95 Gy, p < 0.01). In a study over 169 post-mastectomy breast cancer patients, Li et al. [15] concluded that V20 and V30 of ipsilateral lung were significantly higher (p < 0.001) in the 3DCRT group (32% ± 6% and 22% ± 5%) than in the IMRT group (29% ± 2% and 21% ± 2%). Incidence of grade ≥2 radiation pneumonitis was 23.1% in 3DCRT group versus 6.4% in IMRT group, but this was not statistically significant [17]. Beckham et al. [18] developed eleven-beam, conformal, inverse-planned IMRT plans and compared with standard plans and concluded that IMRT significantly improved volume of lung receiving more than 20 Gy (V20 left lung, 17.1% vs. 26.6%; p < 0.001).
Increase in the rates of cardiac morbidity and mortality following PMRT has always been an area of significant concern. It has been established that adjuvant RT to left sided breast cancers has a small but significant increase in the risk of both cerebrovascular and cardiac deaths [22-24]. Clinical effects of radiation induced heart disease have been observed with therapeutic doses of ≥35 Gy to partial volumes of the heart. There is potentially no threshold dose below which risk of cardiotoxicity does not exist. However, development of cardiotoxicity is a complex phenomenon and depends on a number of conditions like pre-existing cardiovascular diseases, obesity, hypertension, smoking, old age, and use of cardiotoxic chemotherapeutic agents such as anthracyclines, paclitaxel and trastuzumab. Therefore, it is intended that the irradiated heart volume be minimized to the greatest possible degree without compromising the target coverage.
In the present study, the mean dose V25 and V45 for heart were significantly lower in IMRT compared to 3DCRT (4.57 Gy vs. 8.96 Gy, 4.59% vs. 9.19%, and 1.85% vs. 7.09%, respectively; p < 0.001). Gagliardi et al. [25] reported that coronary artery disease risk was much reduced at doses less than Gy. Moorthy et al. [15] concluded that IMRT in comparison to 3DCRT had significantly lower V40 heart (2.13% vs. 7.5%) and Dmax left anterior descending artery (29.17 Gy vs. 39.5 Gy, p < 0.05). Rudat et al. [16] concluded that tangential beam IMRT statistically significantly reduced the V55 by an average of 43% (5.7% vs. 10.6%) and the mean heart dose by an average of 20% (7.04 Gy vs. 8.77 Gy, p = 0.03). Beckham et al. [18] concluded that IMRT significantly reduced volume of the heart receiving more than 30 Gy (V30 heart, 1.7% vs. 12.5%). Cavey et al. [26] concluded that forward planned IMRT technique reduced heart dose and NTCP for each patient at no significant increase in dose to OAR. Smith et al. [27] also concluded that IMRT lowered heart V30 and lung V20. Chang et al. [28] have analyzed 2,577 women from single institute with a median follow-up of 7 years. The mean heart dose was 6.2 and 1.5 Gy for left and right sided tumors. The 10-year cumulative incidence of acute coronary events was 2.96% and the mean time was 5.2 ± 3.9 years (range, 1 to 17 years). There was no clinically relevant difference in rates of acute coronary events between left-sided and right-sided patients, with an adjusted hazard ratio of 1.16 (95% confidence interval, 0.59–2.29).
In the present study, the low dose volume, V5 was significantly higher for IMRT compared with 3DCRT for both the lung (61.48% vs. 51.05%, p < 0.001) and the heart (31.02% vs. 23.27%, p < 0.001). Beckham et al. [18] concluded IMRT increased the volume of normal tissues receiving low-dose RT: V5 right lung (13.7% vs. 2.0%), V5 right breast (29.2% vs. 7.9%), and V5 normal high tissue volume (31.7% vs. 23.6%) (all p < 0.001). Li et al. [17] concluded that V5 of ipsilateral lung was significantly lower (p < 0.001) in the 3DCRT group (52% ± 7%) than in the IMRT group (65% ± 9%); V10 was similar for both groups (41% ± 7% vs. 44% ± 4%). This may translate into secondary malignancies in long term. Hall and Wuu [29] has suggested an increase in incidence of secondary cancer from 1% in conventional planning to 1.75% in IMRT planning for patient’s surviving 10 years. We have to wait long to reach a firm conclusion on this.
In conclusion, IMRT for the irradiation of the chest wall in post-mastectomy left sided breast cancer patients offers the potential to significantly reduce the mean dose and high-dose volumes of the ipsilateral lung and heart compared to 3DCRT (p < 0.001), but 3DCRT remains superior in terms of low dose volume (p < 0.001). IMRT significantly improves the conformity of the plan (p < 0.001) but the homogeneity remains similar with the two techniques (p = 0.83). Now the consequences of higher low-dose volumes with IMRT need to be weighed against the benefits of reducing high-dose volumes on individual patient selection basis. Retrospective nature, less number of patients and short follow-up are the major limitations of the present study. Ideally, the IMRT plan should have been generated for 64 patients who were treated with 3DCRT and 3DCRT plan should have been generated for 43 patients treated with IMRT, then a comparison of two radiotherapy plans should have been done objectively; because every patient has different anatomy and target volumes which could affect the dosimetric parameters. However, due to a very busy set-up, this was not possible, which is one of the major limitations of the present study.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to thank Mr. Ananth Kaliyamoorthy, Consultant Medical Physicist and Radiological Safety Officer, Linear Accelerator Centre, SMS Hospital, Jaipur, Rajasthan, India; for his help and guidance in understanding dosimetry of the treatment plans.