Patterns of care and treatment outcomes for primary thyroid lymphoma: a single institution study
Article information
Abstract
Purpose
The aim of this study was to analyze the patterns of care and treatment outcomes in patients with primary thyroid lymphoma (PTL) in a single institution.
Materials and Methods
Medical records of 29 patients with PTL treated between April 1994 and February 2012 were retrospectively reviewed. Diagnosis was confirmed by biopsy (n = 17) or thyroidectomy (n = 12). Treatment modality and outcome were analyzed according to lymphoma grade.
Results
The median follow-up was 43.2 months (range, 3.8 to 220.8 months). The median age at diagnosis was 57 years (range, 21 to 83 years) and 24 (82.8%) patients were female. Twenty-five (86.2%) patients had PTL with stage IEA and IIEA. There were 8 (27.6%) patients with mucosa-associated lymphoid tissue (MALT) lymphoma and the remaining patients had high-grade lymphoma. Patients were treated with surgery (n = 2), chemotherapy (n = 7), radiotherapy (n = 3) alone, or a combination of these methods (n = 17). Treatment modalities evolved over time and a combination of modalities was preferred, especially for the treatment of high-grade lymphoma in recent years. There was no death or relapse among MALT lymphoma patients. Among high-grade lymphoma patients, 5-year overall survival (OS) and 5-year progression-free survival (PFS) were 75.6% and 73.9%, respectively. Complete remission after initial treatment was the only significant prognostic factor for OS (p = 0.037) and PFS (p = 0.003).
Conclusion
Patients with PTL showed a favorable outcome, especially with MALT lymphoma. Radiotherapy alone for MALT lymphoma and chemotherapy followed by radiotherapy for high-grade lymphoma can be effective treatment options for PTL.
Introduction
Primary thyroid lymphoma (PTL) is a rare malignancy with an incidence of 1% to 5% of all thyroid malignancies and 1% to 2% of all extranodal lymphomas [1,2]. In Korea, its prevalence is decreasing to 0.1% of all thyroid carcinoma as the detection of papillary microcarcinoma is increasing [3].
PTL is more common in mid- to older-aged women, with a peak incidence in the late 60s. The majority of PTLs are non-Hodgkin lymphoma of B cell origin; diffuse large B cell lymphoma (DLBCL) is the most common histologic subtype followed by mucosa-associated lymphoid tissue (MALT) lymphoma [4,5].
Chronic lymphocytic thyroiditis (Hashimoto thyroiditis) is associated with development of PTL, particularly MALT lymphoma [5-7]. MALT lymphoma generally arises in lymphoid tissue as a result of chronic inflammation, but the exact mechanism of malignant transformation from Hashimoto thyroiditis to MALT lymphoma is unknown. High-grade lymphomas including DLBCL, T-cell lymphoma, and Burkitt lymphoma show a more aggressive clinical course than low-grade lymphomas such as MALT lymphoma.
Radiotherapy (RT) alone provides an excellent outcome for MALT lymphoma of the conjunctiva and stomach, and combination chemotherapy (CTx) followed by consolidation RT is standard for high-grade lymphoma [8]. However, no standard treatment is established for PTL and there is a lack of randomized or prospective studies focusing on treatment outcome of PTL due to the rarity of the disease.
In this study, we aimed to analyze the patterns of care during the last two decades and treatment outcomes in patients with PTL in a tertiary hospital setting.
Materials and Methods
Thirty-six patients with PTL who were treated in Severance Hospital between April 1994 and February 2012 were investigated retrospectively. Seven patients were excluded as they had double primary cancers, refused treatment, and died before response evaluation, leaving a total of 29 patients for retrospective analysis.
Because of the low accuracy of diagnosis with fine-needle aspiration (FNA), diagnosis was confirmed by thyroid biopsy or thyroidectomy. The Ann Arbor staging system was used for clinical staging; stage IE, disease confined to the thyroid; stage IIE, disease confined to the thyroid gland and regional lymph nodes above the diaphragm; stage IIIE, disease confined to the thyroid and lymph nodes above and below the diaphragm and/or spleen; and stage IV, disseminated nodal and/or additional extranodal involvement.
Tumor responses were evaluated using updated response criteria from the International Harmonization Project, incorporating positron emission tomography (PET), immunohistochemistry, and flow cytometry for high-grade lymphoma. Complete remission (CR) was defined as complete disappearance of all detectable evidence of disease; PET was negative regardless of residual tumor size in patients whose PET scan was positive before treatment [9]. Responses after initial treatment were described in patients who did not receive total thyroidectomy and relapse/progression was evaluated in all patients. Relapse/progression was defined as the reappearance of disease after CR, the appearance of new disease, or an increase in tumor size. Toxicity was graded using the Common Terminology Criteria for Adverse Events ver. 4.0.
Overall survival (OS) was defined as the time between the diagnosis and death or last follow-up for surviving patients and progression-free survival (PFS) was defined as the time from the end of treatment to the time of disease progression in patients evaluable for relapse/progression.
Statistical analysis was performed using SPSS ver. 20.0 software (IBM, Armonk, NY, USA). OS and PFS were calculated using the Kaplan-Meier method. A p-value <0.05 was considered statistically significant.
Results
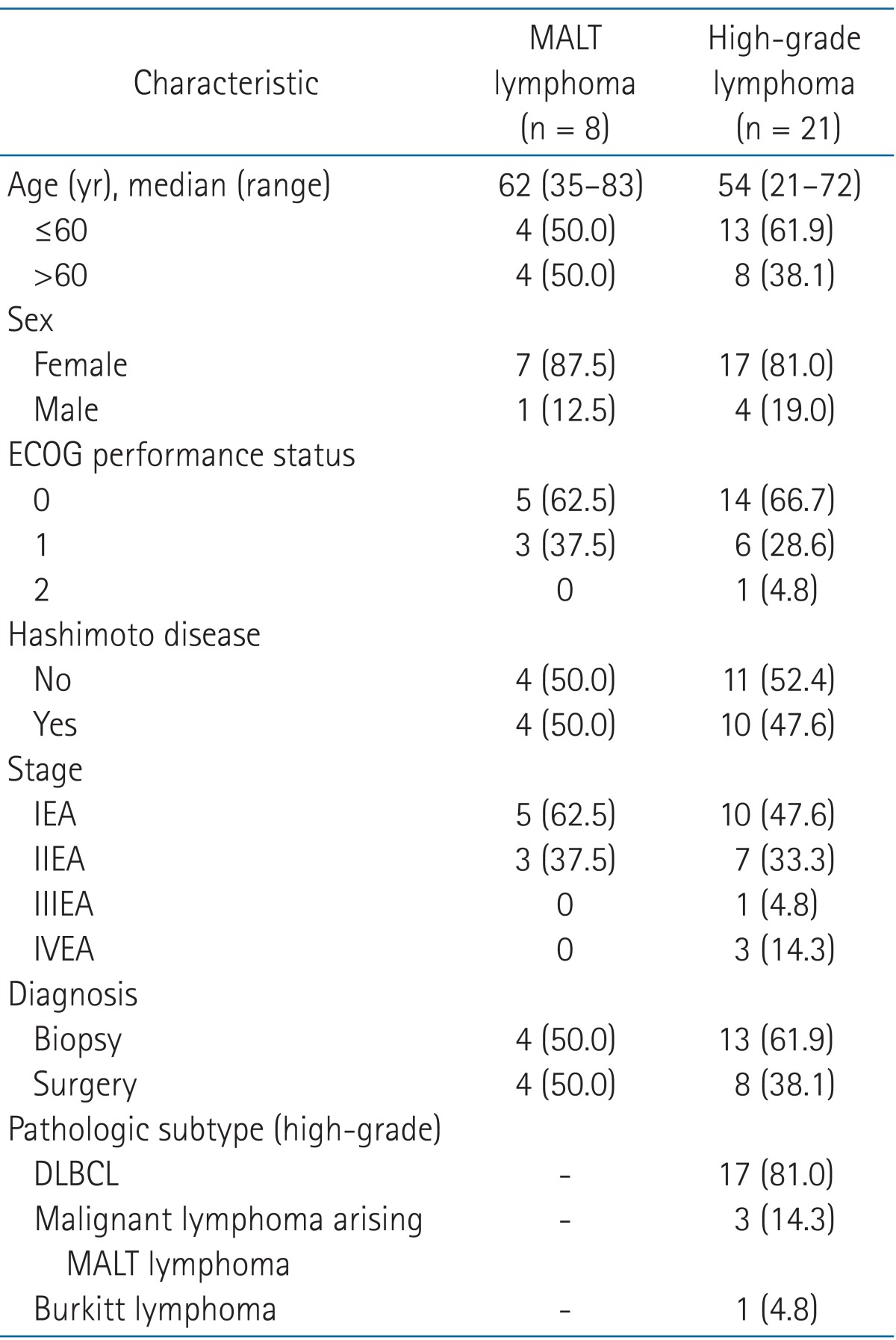
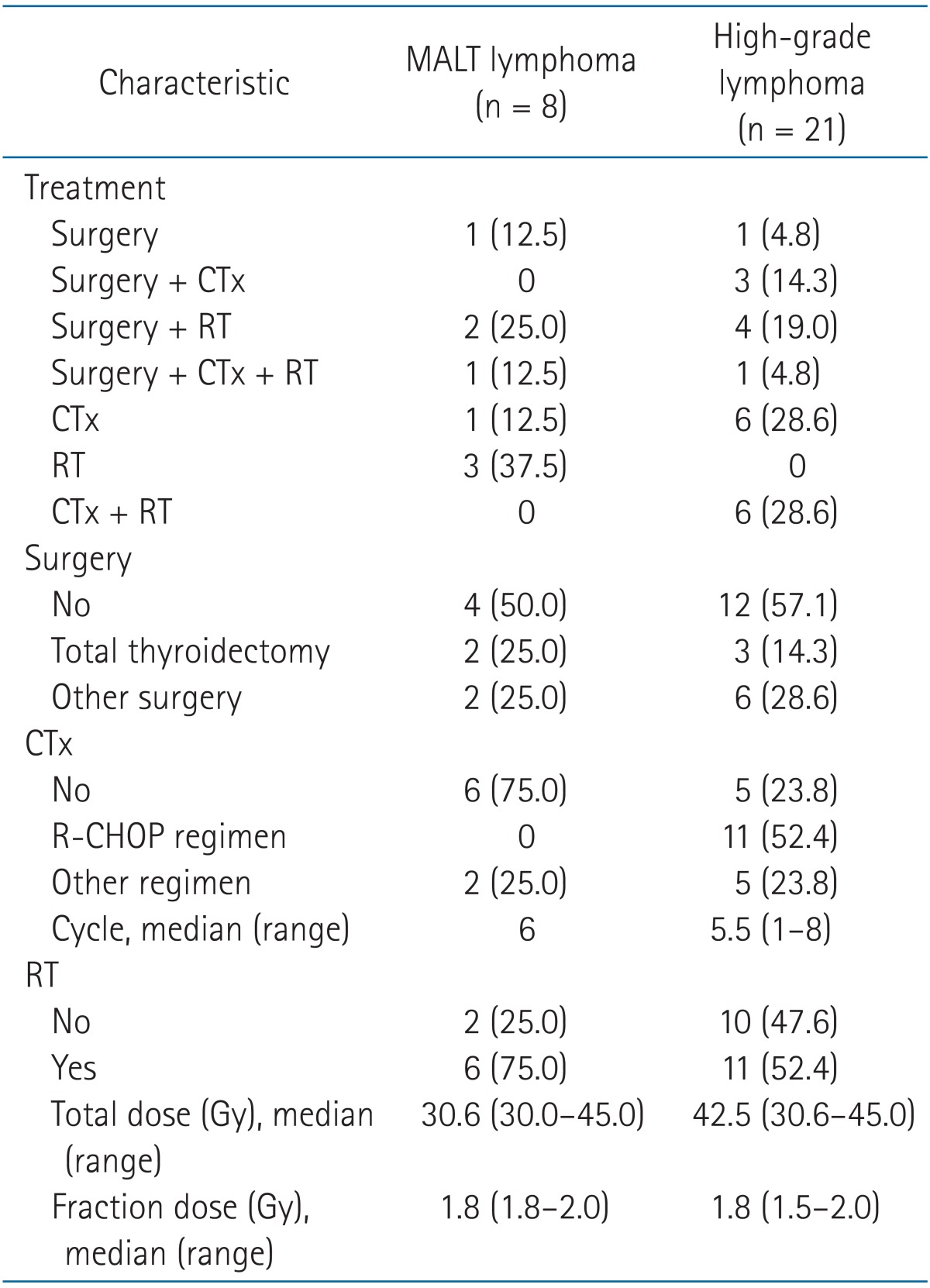
The median follow-up for all patients was 43.2 months (range, 3.8 to 220.8 months). Eight (27.6%) patients were diagnosed as MALT lymphoma and 21 (72.4%) were diagnosed as high-grade lymphoma. The patient and tumor characteristics are shown in Table 1, and the treatment characteristics are described in Table 2. Fig. 1 summarizes initial treatments administered to patients with MALT lymphoma and high-grade lymphoma and treatment response.
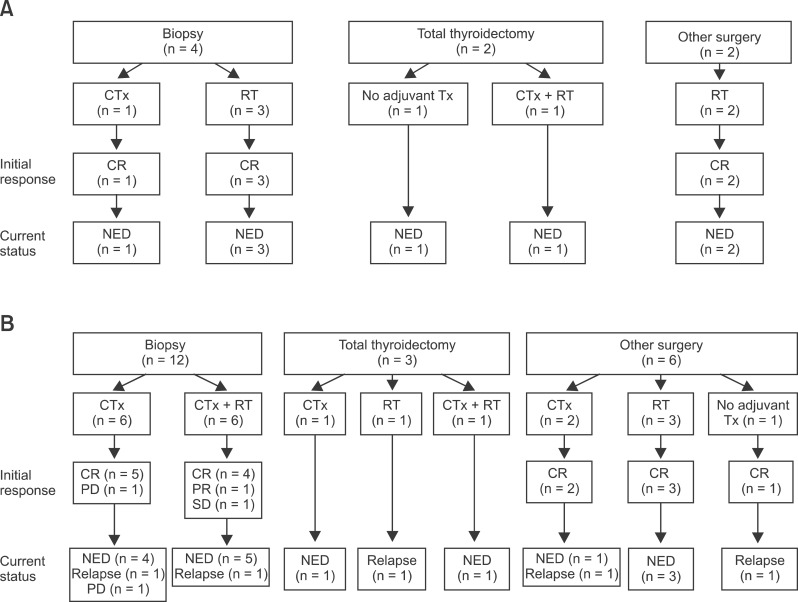
Patterns of care and treatment outcome of mucosa-associated lymphoid tissue (MALT) lymphoma (A) and high-grade lymphoma (B). Treatments were heterogeneous, but diagnosis by biopsy and chemotherapy and/or radiotherapy has been the mainstay in recent years. CTx, chemotherapy; RT, radiotherapy; CR, complete remission; Tx, treatment; NED, no evidence of disease; PD, progression disease; SD, stable disease.
1. MALT lymphoma
The median age of patients with MALT lymphoma at diagnosis was 62 years (range, 35 to 83 years) and 7 (87.5%) patients were female. Four of eight patients (50%) had clinical or histological evidence of Hashimoto thyroiditis. The most common symptom in patients was palpable or growing thyroid mass and no patients in this study presented with B symptoms.
All patients had early-stage lymphoma and there were 5 and 3 patients with stage I and II, respectively.
Four patients (50%) underwent surgery as primary treatment, and among these patients, 1 patient received no adjuvant treatment after total thyroidectomy and three other patients received RT or CTx followed by RT for adjuvant treatment. Four patients were diagnosed with biopsy and treated with RT or CTx alone. The median total dose was 30.6 Gy (range, 30.6 to 45 Gy). A dose of 45 Gy was prescribed in 1 patient who was diagnosed as high-grade MALT lymphoma. The RT volume included the thyroid and involved node area. Prophylactic irradiation to the bilateral neck node or upper mediastinum was done in most patients. Two patients with stage I received radiation to the upper mediastinal node area and only 1 patient received radiation to the thyroid alone. CTx was used in 2 patients.
Treatments have changed over time. In recent years, surgery has not been the main diagnostic and therapeutic tool; RT after diagnosis confirmed by thyroid biopsy has been the main treatment. In 2 patients treated with surgery after 2006, surgery was palliative, not diagnostic, to relieve the pressure symptom due to the thyroid mass, and lymphoma was diagnosed in the pathology specimen.
Treatment outcome in patients with MALT lymphoma was excellent (Fig. 1A). Except 2 patients who received total thyroidectomy, other 6 patients showed complete response after initial treatment. During follow-up period, 7 patients are alive with no evidence of disease and 1 patient is alive with unknown disease status due to follow-up loss.
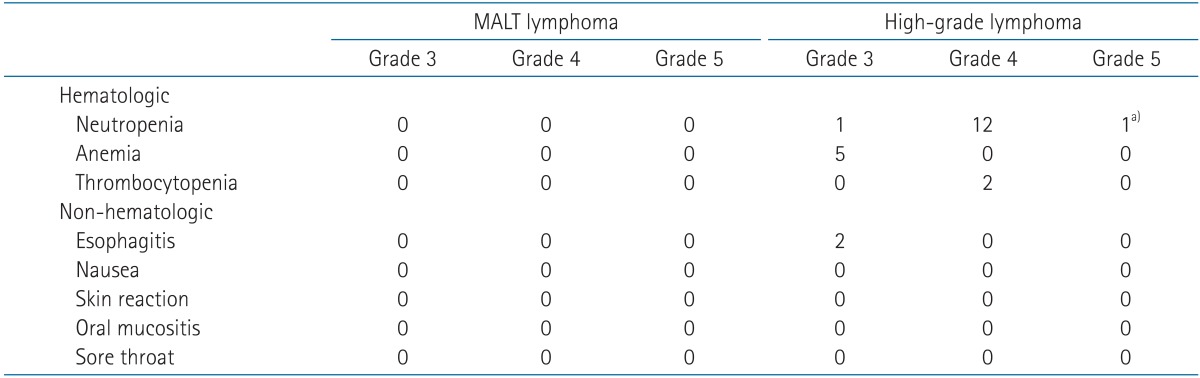
Treatment-related toxicities are shown in Table 3. There was no greater than grade 3 toxicity. Except for 6 patients who were under thyroid hormone replacement therapy before diagnosis of lymphoma or after thyroidectomy, 1 patient had newly diagnosed hypothyroidism at 2 years 3 months after RT.
2. High-grade lymphoma
This group included DLBCL, Burkitt lymphoma, and malignant lymphoma arising MALT lymphoma, and the number of patients in each subtype was 17, 1, and 3, respectively. In 6 patients with DLBCL, MALT lymphoma was mixed with DLBCL. The number of patients with early stage (stage I and II) was 17 (80.9%), and 3 patients were diagnosed as stage IV.
Similar to MALT lymphoma, patterns of care evolved over time for high-grade lymphoma. Before 2006, the mainstay of treatment was surgery, and 8 of 10 patients received thyroidectomy. Surgery in these 8 patients was followed by adjuvant treatment except for the case of 1 patient, and adjuvant treatment included chemotherapy alone (n = 2), RT alone (n = 4), or chemotherapy followed by RT (n = 1). After 2006, only 1 patient received surgery and the other 10 patients were diagnosed with biopsy and received chemotherapy as an initial treatment. RT was given with adjuvant aim after surgery (n = 5) or a consolidation aim after chemotherapy (n = 6). A median of 42.5 Gy (range, 30.6 to 45 Gy) was prescribed and the dose for 6 of 11 patients was 45 Gy. The RT volume was the same as that for MALT lymphoma. Bilateral neck node irradiation was done in all patients regardless of neck node involvement, and 4 patients with stage I received radiation to the upper mediastinum. One patient with stage IVEA gained CR after CTx and received consolidation RT to the thyroid and neck node area after a discussion with a hematologist. Sixteen patients (76.2%) were treated with CTx and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) was the most common regimen.
Initial treatments, treatment response, and current status were shown in Fig. 1B. Except for 3 patients who received total thyroidectomy, CR, PR, SD, and PD after initial treatment were observed in 15, 1, 1, and 1 patients, respectively. Relapse occurred in 5 patients and in 1 patient the disease status was unknown due to death.
The median OS was not reached and OS rates at 5-year and 10-year were 75.6% and 56.7%, respectively (Fig. 2A). Seven (24.1%) patients were dead at the time of analysis and 14 patients were alive without disease. CR after initial treatment (Fig. 3A, p < 0.0001) and no relapse (Fig. 3B, p = 0.014) were statistically significant in univariate analysis. In multivariate analysis, CR after initial treatment was the only significant prognostic factor (p = 0.037). Three of 7 patients died of disease unrelated to lymphoma. Disease-specific survival at 5-year and 10-year was 85.0% and 74.4%, respectively.

(A) Overall survival (OS) and (B) progression-free survival (PFS). Both median OS and PFS not reached. The patients with mucosa-associated lymphoid tissue (MALT) lymphoma were all alive and didn't show a relapse except 1 patient who was lost follow-up. In high-grade lymphoma, 5- and 10-year OS rates were 75.6% and 56.7%, respectively, and 5- and 10-year PFS rates were 73.6% and 63.0%, respectively.
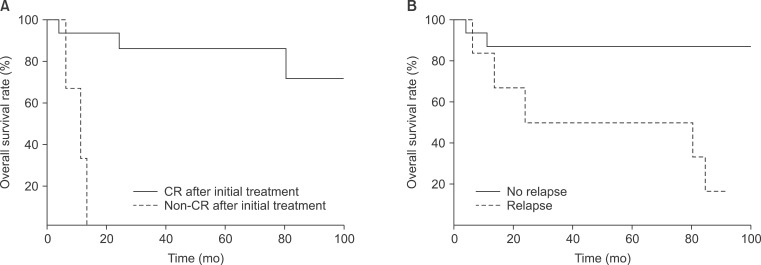
Overall survival (OS) in patients with high-grade lymphoma. (A) Complete remission (CR) after initial treatment was associated with OS (p = 0.039). (B) Patients with a relapse/progression showed worse survival than patients without relapse/progression (p = 0.023).
Relapse after CR or partial response occurred in 4 patients and progression after initial treatment showed in 1 patient. Details of patients with treatment failure are shown in Table 4. One patient was treated with total thyroidectomy and adjuvant RT and relapse on stomach was occurred after 2 years. The median PFS was not reached and 5-year and 10-year survival rates were 73.9% and 63.4%, respectively. Locoregional relapse was the main pattern of relapse. CR after initial treatment was associated with better PFS (p = 0.003).
Salvage treatments were mainly chemotherapy. After salvage treatment, CR was achieved in 3 of 4 patients who could be evaluated for response. One patient was treated in another hospital and the treatment regimen and response were unknown. The patient died 5 months after relapse. Another patient whose final tumor response was unknown had relapsed in the RT field 1 month after completion of RT. Additionally, boost RT of 20 Gy in 10 fractions to the relapse site was given and the palpable neck mass decreased during the treatment. However, further imaging study was not performed due to follow-up loss and she died 9 months after relapse. Two patients who gained CR after salvage treatment died due to pneumonia after autologous stem cell transplantation and progression of vaginal cancer diagnosed after treatment for PTL. Only one patient was alive without disease after relapse.
Toxicity greater than grade 3 was observed in 15 patients, and most cases were hematologic toxicity due to CTx (Table 3). In one patient, neutropenia developed after the second cycle of chemotherapy and the patient died of septic shock. This patient was not included in this study due to death before response evaluation. In 8 patients who were not under thyroid hormone replacement therapy, 2 had newly diagnosed hypothyroidism at 1 year 7 months after CTx, and 4 years 5 months after CTx followed RT.
Discussion and Conclusion
The demographics of our study are consistent with those of previous studies [3,4,10]. PTL was predominant in mid-aged females. Growing thyroid mass was the main symptom and most patients had early stage (stage I and II) in both the MALT lymphoma and high-grade lymphoma groups. DLBCL was the most common histologic subtype.
Although FNA is the first diagnostic investigation in patients with a thyroid mass, its accuracy for PTL has been reported in a wide range, from 25% to 90% [11-13]. Most cases of PTL have been reported to be associated with pre-existing Hashimoto thyroiditis, especially MALT lymphoma. Cytological differentiation of low-grade MALT lymphoma and Hashimoto thyroiditis is difficult and a sampling error can yield false-negative results [3,14]. Adjunctive techniques, such as immunohistochemical staining, molecular techniques, flow cytometry, or polymerase chain reaction to detect a monoclonal immunoglobulin heavy chain have been proposed to improve the diagnostic rate of FNA. However, tissue biopsy is considered the gold standard for histologic diagnosis in PTL [14]. In the current study, only the patients who were diagnosed with tissue biopsy or thyroidectomy were included in order to remove interference from potential misdiagnosis in determining clinical course of such rare disease as PTL.
Due to the rarity of disease, there has been no prospective randomized trial on the treatment for PTL and the current recommendation on the treatment for PTL depends on histologic subtype [10,15]. For indolent subtype of PTL, single-modality treatment with either surgery or RT was considered where combined modality of chemotherapy and RT was preferred for more aggressive subtypes.
The role of surgery in PTL is still controversial and its use for diagnosis and treatment has decreased over time. Graff-Baker et al. [4] reported that the use of surgery for PTL has declined from 81% of patients in 1973 to 1987 to 61% in 1997 to 2005 in the United States. Meyer-Rochow et al. [16] reported that the proportion of operations on patients with a histopathological diagnosis of PTL has significantly decreased and therapeutic thyroid procedures also decreased significantly during the study period. In the current study, surgery was not the main treatment option after 2006 and non-surgical treatment including CTx and/or RT, depending on histologic subtype, has become the mainstay of treatment in recent years.
Histologic subtype was associated with survival in previous studies [3]. There was no statistical significance in the current study due to the small number of patients, but MALT lymphomas showed more favorable outcome than high-grade lymphomas. All patients with MALT lymphoma obtained CR and they are all alive with no relapse. All 6 cases of relapse/progression occurred in patients with high-grade lymphoma and 4 patients died from progression of disease.
Local therapy such as surgery or RT alone was the preferred treatment for localized MALT lymphoma [10,17,18]. In the current study, treatment outcomes between surgery followed by adjuvant treatment and biopsy followed by definitive RT were not different and severe treatment-related toxicity was not observed. Thyroidectomy is an invasive procedure requiring thyroid hormone replacement. Therefore, RT could be an appropriate option for MALT lymphoma in patients confirmed by thyroid biopsy, and this is a mainstay of treatment in localized MALT lymphoma of thyroid.
The optimal RT dose and volume for PTL has not been determined and varies among different institutions [10,17,19]. Harrington et al. [20] recommend extended field RT (EFRT) to a dose of 40 Gy for stage I PTL and chemotherapy followed by consolidation EFRT to a dose of 40 Gy for stage II-IV disease. Alzouebi et al. [10] reported that a low dose, such as 24 Gy in 12 fractions is sufficient for localized PTL of indolent subtype and CTx followed by RT at higher doses is preferred for high-grade disease. In the current study, the median dose of 30.6 Gy was sufficient to obtain CR in MALT lymphoma. Higher RT dose at a median of 45.0 Gy was prescribed for high-grade lymphoma and 1 of 11 patients treated with RT showed relapse 1 month after completion of RT. One patient with stage IV received RT; she obtained CR after CTx and radiation was delivered only to the thyroid and neck node area after discussion with a hematologist. Relapse was not observed and she was alive at follow-up of 76 months. The RT volume included the thyroid and involved the node area and prophylactic irradiation to bilateral neck node or upper mediastinal node area was done in most patients. Patients with stage I who received radiation to the thyroid, neck, and upper mediastinal node area were treated with surgery followed by RT before 2006. In recent years, the RT volume involved the node area and bilateral neck node for prophylactic aim and treatment, and the upper mediastinal node area was considered when this area involved PTL. One patient with stage I MALT lymphoma was treated with RT alone to the thyroid only and 1 patient with stage II DLBCL received consolidation RT to the thyroid and ipsilateral nock node areas after CTx and no relapse occurred in these patients. Further study is needed to determine the optimal RT dose and volume for PTL.
The combination of CTx and RT for high-grade lymphoma was associated with improved survival in previous reports [10,19,21]. In the current study, patients who showed CR after more than 3 cycles of CTx followed by RT had not relapsed and 1 locoregional relapse occurred in 5 patients who showed CR after chemotherapy alone.
There were no significant differences in survival according to patient age, stage, histologic subtype, or treatment modality, which was associated with survival in previous studies [3,6,9,10]. In the current study, CR after initial treatment was an only significant factor for OS and PFS, emphasizing the importance of initial treatment. A larger prospective trial is warranted for establishing consensus treatment for PTL.
In conclusion, patients with PTL showed a favorable outcome, especially with MALT lymphoma. RT alone can be an appropriate treatment option for localized MALT lymphoma. In high-grade lymphoma of the thyroid, locoregional relapse was a common pattern of failure, thus CTx followed by RT is thought to be more beneficial than CTx alone. CR after initial treatment was a significant factor for survival in high-grade lymphoma.
Notes
No potential conflict of interest relevant to this article was reported.