 |
 |
AbstractPurposeThis study evaluated the treatment effectiveness and proper radiation dose of helical tomotherapy (HT) in spine oligometastases from gastrointestinal cancers.
Materials and MethodsFrom 2006 to 2010, 20 gastrointestinal cancer patients were treated with HT for spine oligometastases (31 spine lesions). The gross tumor volume (GTV) was the tumor evident from magnetic resonance imaging images fused with simulation computed tomography images. Clinical target volume (CTV) encompassed involved vertebral bodies or dorsal elements. We assumed that the planning target volume was equal to the CTV. We assessed local control rate after HT for 31 spine metastases. Pain response was scored by using a numeric pain intensity scale (NPIS, from 0 to 10).
ResultsSpine metastatic lesions were treated with median dose of 40 Gy (range, 24 to 51 Gy) and median 5 Gy per fraction (range, 2.5 to 8 Gy) to GTV with median 8 fractions (range, 3 to 20 fraction). Median biologically equivalent dose (BED, ╬▒/╬▓ = 10 Gy) was 52 Gy10 (range, 37.5 to 76.8 Gy10) to GTV. Six month local control rate for spine metastasis was 90.3%. Overall infield failure rate was 15% and outfield failure rate was 75%. Most patients showed pain relief after HT (93.8%). Median local recurrence free survival was 3 months. BED over 57 Gy10 and oligometastases were identified as prognostic factors associated with improved local progression free survival (p = 0.012, p = 0.041).
IntroductionCancer cells most often reach the bones by hematogenous spread. This occurs predominantly at the axial skeleton more than the appendicular skeleton. The thoracic spine is the most commonly involved site in metastatic disease. Eighty percent of spinal metastases patients have spinal body metastasis [1]. Most patients with bone metastases were effectively treated with palliative irradiation in relieving pain. For pain management of bone metastasis, the most widely used palliative radiation therapy regimen is 30 Gy in 10 fractions [2]. Some reports have suggested that there are a considerable number of re-irradiation cases which have responded initially due to an insufficient radiotherapy dose. The commonly used palliative irradiation dose may be not enough to control spinal bone metastases from hepatocellular carcinoma or pancreatobiliary cancer [3].
Stereotactic body radiotherapy (SBRT) uses hypofractionated radiation delivered to a precise target to maximize cell killing by implementation of high accuracy and reproducibility through image-guided radiotherapy (IGRT) [4]. SBRT is frequently used in the treatment of spinal metastases in addition to early stage non-small-cell-lung-cancer (NSCLC), hepatocellular carcinoma and pancreatic cancer etc [5,6]. Chang et al. [7] presented that SBRT could be safe and feasible when using a 30 Gy/5 fractions dose scheme. Ryu et al. [8] reported that the tolerance of the spinal cord is at least 10 Gy to 10% of the cord volume. Since then, the concept has been commonly used in spine SBRT.
This study evaluated the local control rate after high dose radiotherapy by helical tomotherapy in spine oligometastases from gastrointestinal cancers and tried to find the proper radiation dose. We also tried to reveal whether improved local control would increase the local progression free survival (LPFS) rate and to evaluate secondarily the pain reduction rate of spinal metastasis after radiotherapy.
Materials and Methods1. MaterialsFrom 2006 to 2010, 20 gastrointestinal cancer patients (11 hepatocellular carcinomas, 3 cholangiocarcinomas, 2 pancreatic cancers, 2 stomach cancers, 1 colon cancer and 1 rectal cancer) were treated with Helical Tomotherapy (HT, Hi-Art system, Madison, WI, USA) for spine oligometastases at Yonsei Cancer Center, Severance Hospital. We retrospectively analyzed the results based on the clinical record. The patients' ages ranged from 30 to 77 years (median, 60 years) and their characteristics are displayed on Table 1. Total spine lesions were 31 (median 1; range, 1 to 3). Involved spine levels in target volume were in total 78 (6 cervical, 42 thoracic, 24 lumbar, and 6 sacrum). No patient had undergone any spine surgery before radiotherapy. We assessed local control rate and pain response for spinal metastasis lesions after HT and did not include intra-abdominal lesions of primary site in the treatment target. The Eastern Cooperative Oncology Group (ECOG) scale for performance status was ranged from 0 to 3. In this study, patients with less than 5 metastases regions were 5 (25%). Five patients (25%) showed spinal cord lesions in imaging and one of them (5%) was re-irradiated due to a recurrence of the initial treatment lesions.
2. Therapeutic methodsGross tumor volume (GTV) was defined as the volume of lesions seen by magnetic resonance imaging (MRI) images fused with simulation computed tomography (CT) images. Clinical target volume (CTV) was defined differently according to the location of the cancer. If cancer is located on a pedicle, the CTV includes both the vertebral body and dorsal elements; if on spinous process, the CTV includes only dorsal elements; and if on vertebral body, the CTV includes only the corresponding vertebral body. The planning target volume (PTV) was set equal to the CTV. The spinal cord volume was drawn as the cord at the level of the CTV plus 5-6 mm above or below this region defined. If the tumor was small or if the total number of metastatic regions is less than 5, we augmented the fraction size. If the tumor was big or multiple, we reduced the fraction size and increased the number of fractions. The dose fraction scheme was converted to the 2 Gy/fraction (2-Gy dose per fraction equivalent, ╬▒/╬▓ = 0.87 Gy). The 2-Gy dose per fraction equivalent total dose was less than the maximum tolerance dose in spinal cord (54 Gy) [9]. We assumed the dose constraint of the spinal cord to be at least 10 Gy to 10% of the cord volume if the dose per fraction exceeded 5 Gy. We additionally planned to prevent the occurrence of hot spots with the limitation to 20 Gy dose on the spinal cord. Spine metastatic lesions were treated with a median total dose of 40 Gy (range, 24 to 51 Gy) and median fraction size of 5 Gy (range, 2.5 to 8 Gy) to GTV and with median total dose of 28 Gy (range, 15 to 42.5 Gy) and median fraction size of 3.25 Gy (range, 2 to 6 Gy) to CTV. Median biological effective dose (BED, ╬▒/╬▓ = 10 Gy) was 52 Gy10 (range, 37.5 to 76.8 Gy10) to GTV. Radiotherapy was performed with median 8 (range, 3 to 20) fractions. Pinnacle 6.0 (Phillips, Ditchburg, WI, USA) and Tomotherapy planning station (Hi-art System; Tomotherapy, Madison, WI, USA) were used for treatment.
During radiotherapy, a Head-neck-shoulder thermoplastic immobilized system (Type-S; Medtec, Alton, IA, USA) was used if the targets were cervical lesions and a BodyFix system (Medical Intelligence, Schwabmunchen, Germany) was used if the targets were thoracic, lumbar or sacral lesions in treatment planning and process. During Tomotherapy, radiotherapy was done after the correction everyday using megavoltage CT (MVCT) images that were fused with treatment-planning CT images.
3. Determination of treatment effectivenessDuring treatment, we observed and assessed the process of the treatment through weekly meeting with patients. We followed up with the patients every month, every three months and every six months after the end of treatment and thereafter we followed up every six months. We defined the case as being more than a 20% increase in the initial size of the tumor to progressive disease on CT or MRI images after treatment. Local progression free survival was defined as the point of local progression after the end of radiotherapy.
We measured the pain response (no pain, 0; worst pain imaginable, 10) scored by using a Numeric Pain Intensity Scale (NPIS, from 0 to 10). After Tomotherapy, if the pain was reduced to 0 we defined it as complete relief and if there was reduction of more than 3 points we defined it as partial relief. The opioid dose was compared with the opioid dose equivalence conversion table according to the standard National Comprehensive Cancer Network (NCCN) guidelines for adult cancer pain [10].
Toxicity caused by radiotherapy was rated using the National Cancer Institute's Common Terminology Criteria for Adverse Event (CTCAE) ver. 4.0 on a scale.
SPSS ver. 17 (SPSS Inc., Chicago, IL, USA) and the Kaplan-Meier method, respectively, were used for statistical analysis and survival analysis. Logistic regression was used to find the correlation between the radiation dose and local control rate. To determine prognostic factors that govern survival, a log rank test was used for univariate analysis and Cox regression was used for multivariate analysis.
Results1. Treatment effect and treatment failure patternThe median follow-up period of 20 patients was four months (range, 1 to 60 months). Within six months after treatment, there was local progression in 3 out of 31 treatment regions. Therefore, the local control rate was 90.3%. Within the follow-up period (total 180 months), 3 patients out of 20 (15%) had infield disease progression and 15 patients (75%) had outfield disease progression. Infield and outfield treatment failure patients were 3 (15%). The most common distant metastasis regions were bone and lung (8 patients each).
LPFS was 3 months (Fig. 1) in all patients. Progression free survival (PFS) was median 2 months and LPFS rate was 27.8% for 6 months and LPFS was 22.2% for 1 year. The main treatment failure pattern was outfield failure. Success or failure rates of local control do not statistically significantly affect the PFS (p = 0.144) (Fig. 2).
2. Local failure analysisLocal failure was not observed in patients treated more than a 57 Gy10 dose based on BED. Within 6 months, local recurrence was seen in the three infield patients. One of them was treated with 48 Gy10 and the others were treated with 56 Gy10 and all the three patients had spinal cord compression on imaging.
3. Local progression free survival analysisUnivariate analysis was used to determine the prognostic factors governing the LPFS rate (Table 2). The patients were divided according to their ages, one group being those over 60 and the other group younger than 60. There was no significant difference (p = 0.457) in the LPFS rate between the two groups. We confirmed that the primary site was not affected by the LPFS rate (p = 0.906) based on the primary tumor site. If there was a spinal cord compression, the LPFS rate tended to be lower (p = 0.088). Oligometastases with less than 5 metastasis regions had a significantly higher LPFS rate (p = 0.041) than non-oligometastases. We analyzed the infield tumor size with divided diameter by 3 cm. Compared to less than 3 cm, the tumor size of more than 3 cm had a higher statistically significant LPFS rate (p = 0.036). Whether the treatment region was single or multiple did not significantly affect the LPFS rate (p = 0.130). We divided the size of radiation dose per fraction at 5 Gy and analyzed the LPFS rate, but there was no significant difference (p = 0.438). A statistically significant higher LPFS rate (p = 0.012) (Fig. 3) was seen in the group of BED10 (Ōēź57 Gy10) and this led to an increase in the disease-free survival period (p = 0.048) (Fig. 4). There was no valuable prognostic factor in multivariate analysis (Table 3).
4. Pain reductionOf 16 patients experiencing pain, 15 had been given an opioid prior to treatment. When the opioid dose was compared with the opioid equivalence conversion table according to the standard NCCN guidelines for adult cancer pain [10], 3 of the patients (20%) were opioid-free, 6 (40%) used a lower dose than at pre-treatment, 4 (26.7%) used the same dose as pre-treatment, and 2 (13.3%) began using or used more opioid. All 16 patients with pain (NPIS median 7) showed pain reduction, 4 (25%) showed complete pain relief, and 12 (75%) showed partial pain relief. The median value of pain reduction was NPIS five. Pain reduction effects were observed within two months after treatment in 14 patients except two.
5. Treatment-related toxicityDue to acute toxicity, there was one CTCAE grade 1 anorexia patient and two CTCAE grade 1 vomiting patients. After one month of treatment herpes zoster had occurred in one patient. No chronic toxicity was observed. No patient had radiation-induced spinal cord complications after treatment.
Discussion and ConclusionThe recent development of radiotherapy equipment has facilitated the application of hypofractionated radiotherapy and attempts to determine proper treatment volumes and radiation doses are underway in various organizations [11-13]. In palliative radiation therapy of spinal metastases, with conventional external beam therapy, the treatment volume usually encompasses one normal vertebra above and below the metastatic lesions. But high-dose hypofractionated IMRT on spinal metastases has a narrower treatment volume than the existing volume. Despite our treating only the spine with metastastatic lesions defined as PTV in this study, there was no patient with treatment failure around the spine above or below one level of target lesions during the follow-up period. It seems appropriate that the target is limited only to the spine with metastasis lesions.
When SBRT is used on spinal metastasis, to determine the treatment volume and radiation dose we have to consider the following factors: histological findings, the extent or number of involved spinal lesions, the progress of the cancer and the shape and size of the tumor. If the radiation dose per fraction is higher, the dose on the spinal cord should be carefully limited [14]. A lesion near the spinal cord is necessary to increase radiation dose in a safe method, paying attention to the tolerance dose. We could consider using high-precision radiotherapy equipment like Tomotherapy in this case [6]. Tomotherapy could allow a steep dose gradient (10% dose reduction per 1 mm) and protect the spinal cord within a few millimeters of the target.
Tomotherapy is a type of IMRT. It is similar to a helical CT scanner in the rotation of its accelerator and gantry while the patient is moved through a donut-shaped gap and it can treat multiple lesions simultaneously at an average of 10.7 minutes within a short time [15,16]. Also, the tumor can be treated even if it is located within 5 mm of the spinal cord because pre-treatment MVCT can reproduce the patient positioning and can deliver radiation exactly [6,14]. Mahan et al. [17] reported that re-irradiation was successfully performed by HT in spinal metastasis patients with local recurrence and HT was able to maintain a setup error within 1.2 mm without the use of special stereotactic immobilization.
Recently, interest in oligometastases has been increasing. Though oligometastases is a form of distant metastatic progression, it has been shown that the progress of the tumor in the metastatic region can be prevented or delayed by active local treatment such as surgery or radiotherapy. This is known to improve the survival rate ultimately through improved local control in this state [18]. Higher BED hypofractionated radiotherapy in oligometastases has been attempted. Milano et al. [19,20] announced that the local control rate and the survival rate were improved when hypofractionated radiotherapy was performed in limited metastases (defined as five lesions or fewer). Inoue et al. [21] also reported that the 3-year local control rate increased up to 80% after SBRT to oligometastases. Our center commonly uses the 24 Gy/3 fractions dose that is regarded as safe considering the spinal cord tolerable dose [9] when we treat patients with spinal metastases by Tomotherapy. This is equivalent to 33 Gy/11 fractions corresponding palliative radiation therapy if it is converted to BED (╬▒/╬▓ = 10 Gy). If we used this dose scheme, the local control rate was quite low by 39% according to the results of previous studies. Dose increment would be needed for oligometastases patients who may have survival improvement by local control [18,20].
After radiotherapy, it was reported that bone metastases of gastrointestinal cancers showed response rates similar to those of bone metastases from other primary cancers [22]. According to the study of Milano et al. [19], a 4-year 73% high local control rate was seen when 50 Gy/10 fractions was used for treatment of oligometastases. Among gastrointestinal cancers, pancreatobilliary cancer or hepatocellular cancer was reported to have a respectively lower local control rate compared to other primary cancers [23]. Other research has shown that image response and pain reduction increased by the use of high-dose radiotherapy (39 Gy10) on hepatocellular cancer [24].
In this study, radiation dose was a factor that affected local control. The 24 Gy/3 fractions dose commonly used in spinal metastases is equivalent to 43.2 Gy10 if it is converted to BED (╬▒/╬▓ = 10 Gy) and this dose is also equivalent to 30 Gy/5 fractions, 33 Gy/11 fractions and 36 Gy/18 fractions. This is not a curative-intention therapy but rather a palliative treatment radiation therapy.
All three patients who failed the 6 month-based local control have spinal cord compression on imaging and were treated with BED 48 Gy10 or 56 Gy10. They had complained of motor weakness before radiation therapy. These patients had larger-sized tumors inside the spinal canal after treatment. One of them with worsened symptom showed paraplegia at the end of treatment. The patient already had grade III motor weakness before treatment. Radiation therapy seemed to be unable to prevent the worsening of neurological symptoms because the symptoms were already present from the start of treatment. The local control rate with spinal cord compression seemed to be worse than others. For these patients, respectively lower dose, thought to be safe, was selected due to the dose limit to adjacent damageable organs, i.e. the spinal cord. It seemed not to be an effective dose to local control of the tumor. When the life expectancy of patients is less than 6 months, the improvement of the local control rate by radiation exceeds the dose limit of the spinal cord because the spinal cord is a late responding organ. However, if the patients survive beyond their life expectancy, a very small number of patients may have the risk of severe side effects like radiation myelitis. Therefore, surgery or other methods of therapy than SBRT should be considered as a priority to patients with spinal cord compression. One of the local failure patients received re-irradiation due to the recurrence of the same lesions. When radiation therapy was used as a conventional treatment, a total dose of 38 Gy (initial dose of 5 Gy in 2 fractions followed by the dose of 33 Gy in 11 fractions) was irradiated and after 9 months 30 Gy (5 Gy dose per fraction) was re-irradiated by Tomotherapy and the maximum radiation dose for the spinal cord was limited to less than a total of 20 Gy dose. Re-irradiation data in animals and humans shows that partial repair of radiotherapy (RT)-induced sub-clinical damage becomes evident about 6 months after RT and increases over the next 2 years [9]. This patient had no post-RT side effects and showed tumor size increment of irradiated lesion after 3 months of RT. It may be thought that it didn't respond to treatment because radiation-resistant cells were redistributed [25,26].
Of the patients studied, two patients with hepatocellular oligometastases and currently with no recurrence or metastases were in successful local control after treatment. The size of tumors was small and all lesions were included in the treatment area. The patients were treated using 48 Gy/8 fractions (╬▒/╬▓ =10 Gy, BED 76.8 Gy10) of high-dose radiation (Fig. 5). We knew that the 50 Gy/10 fraction dose used in the Milano et al. [19]'s study with a 2-year 77% local control rate was equal to 48 Gy/8 fractions (BED 75 Gy10). It was a high dose also equivalent to 64 Gy/32 fractions (2 Gy per fraction). These patients showed additional metastases lesions outside the treatment range, but they could be controlled by pertinent treatment. We observed that after radiation, soft-tissue metastases were reduced in size and re-calcified by observation with position emission tomography-computed tomography (PET-CT) images. High-dose radiation to hepatocellular metastases resulted in excellent treatment results [24]. Oligometastases needs to be treated aggressively. In addition, the use of radiation dose escalation and hypofractionated dose in SBRT should be primarily considered.
The results of this study have shown that there was a difference in the local control rate by 3 cm based on the maximum tumor diameter and that the larger size of the tumor does not respond to radiation therapy. This was also shown by Milano et al. [19,20].
It seems that young patients have strong cancer-resistant immune systems [27] and need more active treatment. However, the age of patients was not a significant factor [28] affecting the grade of palliative radiation therapy on bone metastases not only in prior research results but also in additional analysis of this study.
In this study, all 16 patients with pain in the spinal metastases region experienced pain relief after treatment. Because two of them took more opioid analgesics after treatment, it is difficult to say that all patients had effective pain relief. This means that only 14 patients (87.5%) experienced a pain relief effect.
The pain caused by bone metastases occurs mainly by metamorphosis of the periosteum with its multitude of nerves [29] and the pain caused by an unstable spine was known to be around 10%. The determination of a spinal instability was based on clinical judgment, taking the following factors into account: 1) extent of spine collapse; 2) presence of spine deformity; 3) involvement of all three columns of the spine; 4) severe mechanical pain [30]. Pain associated with a spinal instability did not respond well to radiation therapy [31]. There was no statistically significant correlation between pain response and local control (Pearson's Žć2 test, p = 0.751) in this study. Pain was influenced by the site and the shape of the spinal metastases regions and was controlled by respectively lower doses than the required dose for local control and the response for radiotherapy seemed to be favorable [32].
This study was a retrospective analysis and its treatment methods were inconsistent and might have a selection bias. Analysis for prognostic factors was unreliable due to the low number of patients (20). In addition, due to the lack of survival time for most of the patients, the short follow-up period was a limitation to analysis. For clearer results, a large number of prospective clinical studies are required.
In conclusion, if high-dose Tomotherapy (ŌēźBED 57 Gy10) is applied to spinal oligometastases, less than 3 cm and free of spinal cord compression by hypofractionated radiotherapy, there can be a successful local control rate and extension of the LPFS period.
AcknowledgmentsThis research was supported by the grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (0620390).
References2. Foro Arnalot P, Fontanals AV, Galceran JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008;89:150ŌĆō155, PMID: 18556080.
3. Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol 1998;93:2167ŌĆō2171, PMID: 9820391.
4. Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 2011;14:151ŌĆō166, PMID: 21184635.
5. Thariat J, Marcy PY, Lagrange JL. Trends in radiation therapy for the treatment of metastatic and oligometastatic disease in 2010. Bull Cancer 2010;97:1467ŌĆō1476, PMID: 21220224.
6. Song DY, Kavanagh BD, Benedict SH, Schefter T. Stereotactic body radiation therapy. Rationale, techniques, applications, and optimization. Oncology (Williston Park) 2004;18:1419ŌĆō1430, PMID: 15609470.
7. Chang EL, Shiu AS, Lii MF, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys 2004;59:1288ŌĆō1294, PMID: 15275711.
8. Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery 2001;49:838ŌĆō846, PMID: 11564244.
9. Kirkpatrick JP, van de rKogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S42ŌĆōS49, PMID: 20171517.
10. Swarm R, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2010;8:1046ŌĆō1086, PMID: 20876544.
11. Sheehan JP, Jagannathan J. Review of spinal radiosurgery: a minimally invasive approach for the treatment of spinal and paraspinal metastases. Neurosurg Focus 2008;25:E18PMID: 18673047.
12. Yamada Y, Lovelock DM, Bilsky MH. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery 2007;61:226ŌĆō235, PMID: 17762734.
13. Olsen JR, Robinson CG, El Naqa I, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e299ŌĆōe303, PMID: 21477948.
14. Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys 2008;71:652ŌĆō665, PMID: 18514775.
15. Sterzing F, Schubert K, Sroka-Perez G, Kalz J, Debus J, Herfarth K. Helical tomotherapy. Experiences of the first 150 patients in Heidelberg. Strahlenther Onkol 2008;184:8ŌĆō14, PMID: 18188517.
16. Kim B, Soisson ET, Duma C, et al. Image-guided helical Tomotherapy for treatment of spine tumors. Clin Neurol Neurosurg 2008;110:357ŌĆō362, PMID: 18295971.
17. Mahan SL, Ramsey CR, Scaperoth DD, Chase DJ, Byrne TE. Evaluation of image-guided helical tomotherapy for the retreatment of spinal metastasis. Int J Radiat Oncol Biol Phys 2005;63:1576ŌĆō1583, PMID: 16125871.
18. Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat 2009;115:601ŌĆō608, PMID: 18719992.
19. Milano MT, Katz AW, Schell MC, Philip A, Okunieff P. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:1516ŌĆō1522, PMID: 18495378.
20. Milano MT, Philip A, Okunieff P. Analysis of patients with oligometastases undergoing two or more curative-intent stereotactic radiotherapy courses. Int J Radiat Oncol Biol Phys 2009;73:832ŌĆō837, PMID: 18760543.
21. Inoue T, Katoh N, Aoyama H, et al. Clinical outcomes of stereotactic brain and/or body radiotherapy for patients with oligometastatic lesions. Jpn J Clin Oncol 2010;40:788ŌĆō794, PMID: 20406944.
22. Hird A, Chow E, Yip D, et al. After radiotherapy, do bone metastases from gastrointestinal cancers show response rates similar to those of bone metastases from other primary cancers? Curr Oncol 2008;15:219ŌĆō225, PMID: 19008996.
23. Jang JW, Kay CS, You CR, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys 2009;74:412ŌĆō418, PMID: 18963538.
24. Kim TG, Park HC, Lim DH, et al. Radiation therapy for bone metastases from hepatocellular carcinoma: effect of radiation dose escalation. J Korean Soc Ther Radiol Oncol 2011;29:63ŌĆō70.
25. Mahadevan A, Floyd S, Wong E, Jeyapalan S, Groff M, Kasper E. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys 2011;81:1500ŌĆō1505, PMID: 20950944.
26. Garg AK, Wang XS, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer 2011;117:3509ŌĆō3516, PMID: 21319143.
27. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589ŌĆō595, PMID: 19349616.
28. Campos S, Presutti R, Zhang L, et al. Elderly patients with painful bone metastases should be offered palliative radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1500ŌĆō1506, PMID: 19540056.
29. Park HC, Seong J, An JH, Kim J, Kim UJ, Lee BW. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys 2005;61:1523ŌĆō1534, PMID: 15817359.
30. Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151ŌĆō160, PMID: 17688054.
31. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165ŌĆō176, PMID: 11417967.
32. Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965ŌĆō976, PMID: 21277118.
Fig.┬Ā2Progression free survival according to local control. Three patients in local control (-) group and 17 patients in local control (+) group. 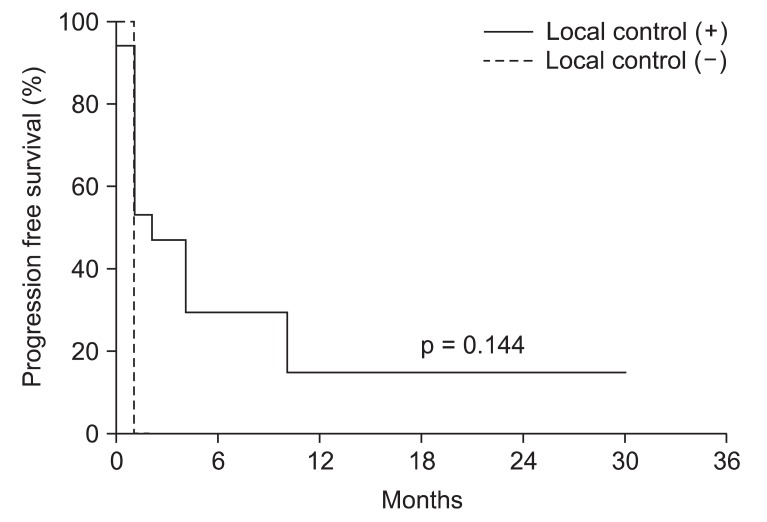
Fig.┬Ā3Local progression free survival rate according to biological effective dose (BED10 57 Gy10) of the Tomotherapy. Six patients in BED10 > 57 Gy10 group and 14 patients in BED10 Ōēż 57 Gy10 group. 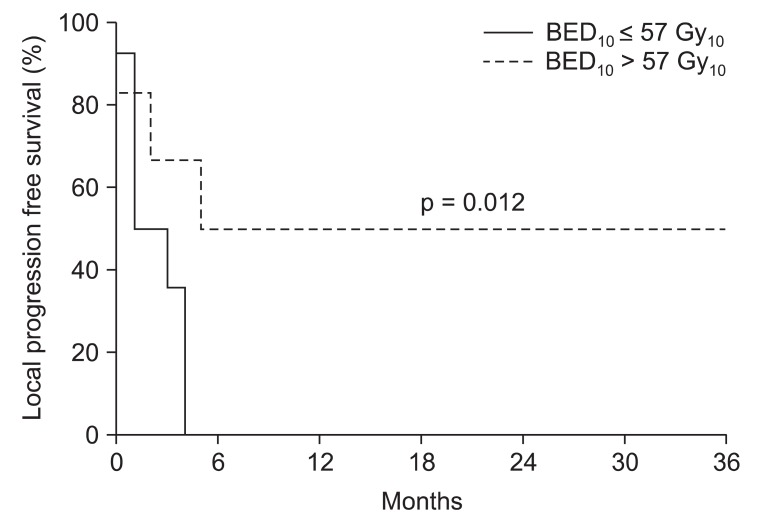
Fig.┬Ā4Progression free survival rate according to biological effective dose (BED10 57 Gy10) of the Tomotherapy. Six patients in BED10 > 57 Gy10 group and 14 patients in BED10 Ōēż 57 Gy10 group. 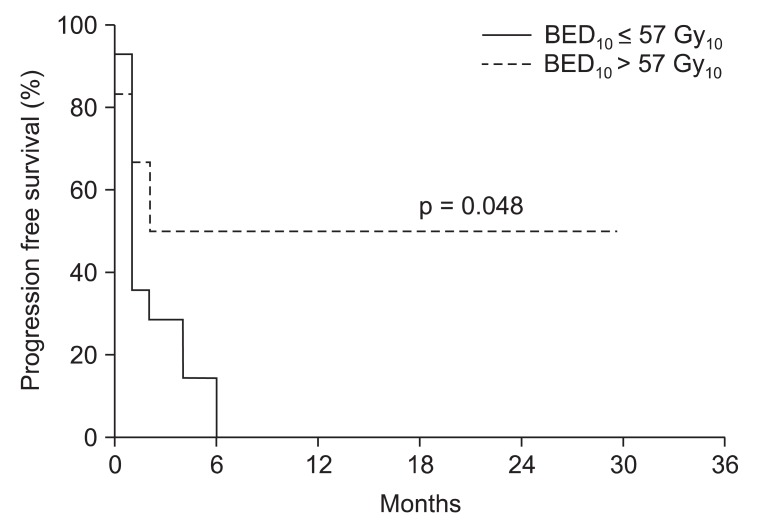
Fig.┬Ā5Illustration of a patient case with hepatocellular carcinoma multiple metastasis in the L1 vertebra body. Positron emission tomography-computed tomography (PET-CT) before Tomotherapy showed hypermetabolic lesion in L1 (A). Prescriptive radiation dose to gross tumor volume (GTV) and clinical target volume (CTV) were 48 Gy and 24 Gy with 8 fractions, respectively (B). On 6 months after Tomotherapy, there was no viable tumor lesion on previous tumor site in PET-CT (C). 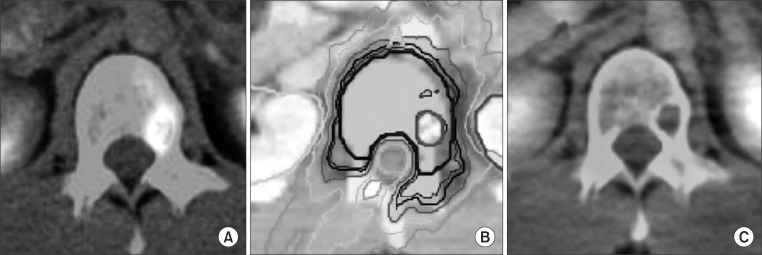
|
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |