 |
 |
AbstractPurposeIn a previous study, the transmembrane protein FXYD-3 was suggested as a biomarker for a lower survival rate and reduced radiosensitivity in rectal cancer patients receiving preoperative radiotherapy. The purpose of preoperative irradiation in rectal cancer is to reduce local recurrence. The aim of this study was to investigate the potential role of FXYD-3 as a biomarker for increased risk for local recurrence of rectal cancer.
Materials and MethodsFXYD-3 expression was immunohistochemically examined in surgical specimens from a cohort of patients with rectal cancer who developed local recurrence (n = 48). The cohort was compared to a matched control group without recurrence (n = 81).
ResultsWeak FXYD-3 expression was found in 106/129 (82%) of the rectal tumors and strong expression in 23/129 (18%). There was no difference in the expression of FXYD-3 between the patients with local recurrence and the control group. Furthermore there was no difference in FXYD-3 expression and time to diagnosis of local recurrence between patients who received preoperative radiotherapy and those without.
IntroductionFollowing the introduction of preoperative radiotherapy (RT) in the treatment of rectal cancer, the rate of local recurrence (LR) has been reduced from incidences of 15%ŌĆō35% to 5%ŌĆō8% in combination with improved surgical technique according to the principles of total mesorectal excision (TME) [12]. Even in combination with optimal surgical technique, RT is still beneficial for the reduction of LRs [3]. There are well-known short- and long-term side effects from pelvic radiation such as urinary and faecal incontinence, sexual dysfunction, peripheral nerve damage, chronic pain, and pathological fractures. Small bowel toxicity with an increased risk for bowel obstruction is probably the most frequent side effect [456]. The incidence of severe radio toxicity with leukopenia, abdominal pain and diarrhoea is around 5% [78]. The late effects of RT may add to the complications of pelvic surgery. Both irradiation and the surgical trauma in the area of pelvic nerves can harm the nerves controlling sexual function and continence of both the bowel and bladder. The increased risk for nerve damage after RT, independent of surgical complications, has been described in several studies [46]. The risk for secondary cancer is increased after irradiation [4]. The aim of preoperative RT is primarily to reduce the risk for LR [39]. Since the current risk for LR with optimal surgery is below 10% even without irradiation, there is a large proportion of patients receiving RT unnecessarily. It is well known from previous studies that the response to RT is highly individual [10], and that this may partly be explained at the molecular level. One example is the absence of functional p53 [11], which is common among rectal cancer patients and reduces radio sensitivity [11]. Apart from p53, p73, survivin and phosphatase of regenerating liver are factors that have an impact on radio sensitivity [121314]. A number of clinically available biomarkers to predict radio sensitivity have been sought after, but so far none has come into clinical use.
Another way to select patients for RT, thus avoiding overtreatment, would be to define risk factors for LR. Potential biomarkers for LR of rectal cancer have been investigated and some are regarded as promising. Examples of such biomarkers are epidermal growth factor, thymidylate synthase and p21 [10].
In a previous study including patients from the Swedish trial on preoperative RT for rectal cancer, we found that a strong expression of the membrane protein FXYD-3 was associated with infiltrative tumour growth and a reduction in tumour necrosis. Tumours with weak FXYD-3 expression had a better prognosis after RT [15].
FXYD proteins have been shown to be tissue-specific modulators of Na+/ K+ ATPase [1617]. FXYD-3 messenger RNA (mRNA) is over-expressed in a variety of human benign and malignant tumours [1718].
To summarize, these results indicate that FXYD-3 may be useful as a biomarker for poor outcome in rectal cancer. Since the primary aim of RT is the reduction of LR, and not increased survival, we investigated the expression of FXYD-3 and clinicopathologic variables in rectal cancer patients with or without LR. Our aim of this study was to see if FXYD-3 is over-expressed in the tumours of patients who later develop LR.
Materials and Methods1. Selection of patientsThe study population comprised patients operated for rectal cancer in Sweden. The cases were extracted from the databases of three healthcare regions with a catchment area of 2.3 million inhabitants: Stockholm/Gotland 1995ŌĆō1999, Uppsala 1985ŌĆō1995, and Norrkoping 1990ŌĆō2000. The same patient population was previously used by Syk et al. [1920] in their study on LR of rectal cancer. In all 3 regions, surgeons trained in rectal cancer surgery including TME, performed the majority of the operations. The majority of patients with RT received short preoperative radiation, 5 ├Ś 5 Gy. In locally advanced tumours patients received long course radiation in doses of 1.8 Gy to a total dose of 45ŌĆō54 Gy over 25 days. The treatment was given with a linear accelerator with energy of 6ŌĆō15 MV of photons using a three- or four-field technique.
During the last few years of the study period some patients with long course radiation could have had concomitant 5-fluorouracil treatment. The use of chemotherapy was sporadic and mainly used in the palliative setting [21].
A total of 1,180 patients were included and operated with radical resection (R0) of rectal adenocarcinoma via an abdominal or abdomino-perineal operation. Forty-eight patients who developed a LR, defined as any recurrence of rectal cancer within the pelvis, were identified from the data set. Patients with concurrent liver- or lung metastases at the time of recurrence were also included. The LR was diagnosed morphologically in 40 of the 48 patients (83%) retrieved, with computed tomography or magnetic resonance imaging in six patients (13%), and by clinical examination alone in two patients (4%). All LRs were anatomically located below the level of S1-S2 in the pelvis. To evaluate the expression of FXYD-3 in LR, a nested caseŌĆōcontrol study was designed. For each case with LR, two control patients from the study population were selected. The control patients were matched for gender and preoperative RT. In each control patient an observation period free from locally recurrent disease at least as long as the matched LR case was required. The median follow-up time for the cases was 79 weeks (range, 56 to 220 weeks) and for the controls 316 weeks (range, 14 to 582 weeks). Eighty-one percent of the control patients had a follow-up exceeding 3 years. One patient in the control group later developed LR. From the cohort of 174 patients (58 cases and 116 controls), it was possible to retrieve 129 (48 cases and 81 controls) formalin-fixed paraffin wax-embedded blocks with tumour from the primary operation.
Baseline data on included patients are presented in Table 1. The regional ethics committees approved the study.
2. ImmunohistochemistryThe original hematoxylin & eosin stain slides were re-examined by a senior pathologist to confirm the diagnosis and the degree of representation.
Immunohistochemistry was performed on 4 ┬Ąm thick formalin-fixed paraffin wax-embedded slides. They were deparaffinized in xylene (2 ├Ś 10 minutes), rehydrated in graded ethanol (99.5%, 95%, 70%, respectively) and washed in distilled water. For antigen retrieval, the slices were boiled in a pressure cooker (2100-Retriever; HistoLab, Gothenburg, Sweden) in Dako Target Retrieval Solution (pH 9.0), and rinsed twice. To quench the endogenous peroxidase activity we used 3% hydroperoxidase in tap water for 20 minutes and rinsed twice. Non-specific background staining was blocked with Dako Protein Block Serum-Free for 10 minutes.
The primary monoclonal antibody was incubated overnight at 4Ōäā 1:10 in antibody diluent (Dako, Glostrup, Denmark) and rinsed twice. After removing the blocking solution, the sections were incubated with a monoclonal anti-FXYD-3 primary antibody (Applied Tumour Virology, Heidelberg, Germany).
Incubation with an amplification system with a labelled polymer/HRP, EnVision Rabbit/Mouse K5007 (Dako) for 30 minutes and also rinsed twice before the peroxidase reaction was performed for 10 minutes in 3,3'-diaminobenzidine tetrahydrochloride (DAB) solution (Dako).
The slices were then rinsed in water. Counterstaining was done with Mayer's hematoxylin for 40 seconds followed by rinsing in tap water at 37Ōäā for 5 minutes and dehydrated in graded ethanol (70%, 95%, 99.5%, respectively) and mounted in xylene-based mounting medium in Pertex (HistoLab).
As positive and negative controls, tissue samples with known strong immunostaining for FXYD-3 were used in each run, using either the primary antibody or universal mouse IgG (Dako). In all runs the positive controls showed obvious staining and the negative controls showed no staining.
The stained slices were microscopically examined and independently scored by two of the investigators blinded to the clinic-pathologic or biologic data. For those disagreed slides, the investigators examined the slides again separately. Finally, 6 uncertain slices were re-examined by dual-head microscopy, and a concurrent score was achieved. The staining and examination processes were performed at the Karolinska Institute, Solna, by two histopathologists. Eighteen randomly selected slices were also examined by an examiner at Linkoping University blinded to the previous scoring at the Karolinska Institute. The scoring was confirmed for all 18 slices.
Staining was graded as negative (grade 0), weak (grade 1), moderate (grade 2), and strong (grade 3), based on the intensity of the staining of the cell membrane or cytoplasm in normal epithelial or tumour cells. The cases with negative, weak and moderate staining (grades 0, 1, and 2) were grouped together as the weakly stained group and the cases with strong staining (grade 3) formed the strongly stained group for statistical analyses. To avoid artefacts, tissues in the areas with poor morphology, necrosis, or in the margins of the sections were not considered.
3. Statistical analysesThe chi-square method was used to test the significance of the differences in FXYD-3 expression between the strong FXYD-3 expression group and the weak FXYD-3 expression group. The figure depicting time to LR was calculated using the Kaplan-Meier method.
The tests were two-sided, and a value of p < 0.05 was considered statistically significant. Logistic regression and Cox regression were used to analyse the effect of differences between the cases and the control group. Statistical analyses were performed using the SPSS ver. 22 (IBM, Armonk, NY, USA) computer program.
ResultsFXYD-3 expression was located in the cell membrane and in the cytoplasm in the normal epithelial cells as well as in the rectal cancer cells. FXYD-3 was evenly distributed throughout the tissues (Fig. 1).
Statistical calculations were performed on both membrane and cytoplasmic expression without any significant difference in the results. Tables and figures are based on cytoplasmic expression. There was no significant difference in tumour FXYD-3 expression between the control group and the LR group (Table 1).
When looking at the time to diagnosis of a LR, there was no significant difference between those with strong FXYD-3 expression and those with weak FXYD-3 expression (Fig. 2). When all patients were analysed together, both those with and without LR, there was no difference in survival in the radiated group between patients with strong or weak FXYD-3 expression (p = 0.43). There was no difference in survival in the unradiated group (p = 0.30) between the patients with strong or weak FXYD-3 expression.
Tables 2 and 3 show FXYD-3 expression in relation to preoperative RT. There was a tendency towards a lower incidence of strong FXYD-3 expression in patients with LR treated with preoperative RT (Table 3). However, because of the small number of observations no significant differences were observed. We have also performed a regression analysis with the included variables (Tables 1 and 4).
Discussion and ConclusionFXYD-3 is known to be expressed in a variety of benign and malignant tumours such as precancerous adenoma of the pancreas and in early malignant androgen-dependent prostatic cancer. This suggests that FXYD-3 is up-regulated early in the process of malignant transformation [1718]. Meding et al. [22] found that FXYD-3 acts as a proteomic marker for lymph node metastasis in colorectal cancer. It has been shown that the expression of FXYD-3 is evenly distributed in tissues making it suitable as a biomarker [15]. In a previous immunohistology study we found significantly increased expression of FXYD-3 in rectal cancer compared to normal mucosa. We could also see a positive correlation between strong FXYD-3 expression and infiltrative growth pattern, and reduced tumour necrosis after RT. In a group of nonirradiated rectal cancer patients there was no correlation between FXYD-3 expression and overall survival, but in in a group receiving RT there was a significant relation between strong FXYD-3 expression and decreased overall survival rate [15]. Our conclusion in that study is that patients with strong FXYD-3 expression who receive preoperative RT have a similar clinical outcome as nonirradiated patients whereas irradiated patients with weak FXYD-3 expression do better. Since we had previously seen reduced tumour necrosis in the irradiated group with strong FXYD-3 expression, we hypothesized that strong FXYD-3 expression could be a biomarker for reduced radio sensitivity [15]. We also suspected that FXYD-3 might function as a general marker for rectal cancer outcome.
The major purpose of preoperative RT is to reduce LR [239]. To confirm our earlier findings we have now studied a cohort of patients who developed LR. This population-based cohort of patients is large compared to other reports in the current literature. We expected to find a higher incidence of strong FXYD-3 expression in tumours that later led to LR after preoperative RT. Our results, however, showed a tendency towards a lower incidence of strong FXYD-3 expression in the RT group with LR, but the numbers are small and the difference was not significant. The results did not show any correlation between the strong FXYD-3 expression and the time to diagnosis of LR. We acknowledge that there were differences between the control group and LR group in both T-stage and tumour differentiation (Table 1). In order to further analyse the effect of the differences in degree of differentiation and tumour stage between the LR group and the control group we used a logistic regression analysis with LR as outcome variable and tumour stage and differentiation as predictor variable. No significance was found for FXYD-3 expression. Neither could a Cox regression analysis, with time to recurrence as outcome variable, demonstrate any correlation between FXYD-3 and time to recurrence when compared with the other variables.
We also acknowledge that the proportion of patients without RT in combination with a small proportion of tumours with strong FXYD-3 expression makes it hard to achieve significant differences. The extent of FXYD-3 expression in the tumours could not be anticipated. This was a study regarding surgical specimen. Using preoperative biopsies for immunohistochemistry could have been an option but would have required more tumour tissue than was available after the pathology assessment of the biopsies.
Different ways of grouping FXYD-3 expression including grades 0 + 1 as weak group and grades 2 + 3 as strong group did not alter the results. Therefore the initial grouping was used in the statistical analyses. It is logical to use the development of LR as a marker for radio sensitivity, although other factors (quality of surgery and tumour characteristics) also play a part.
In the light of the conflicting results regarding FXYD-3 and prognosis of various malignant diseases, we believe that the results of the present study add to our current knowledge of this protein. We could not show it to be a useful biomarker for the risk of developing LR. To our knowledge no other study has investigated the relationship between FXYD-3 expression and LR in rectal cancer patients.
AcknowledgmentsThe authors thank Marja Hallstrom, Karolinska Institute, Stockholm for her skilful laboratory assistance and Dr. Peter Cox in Vrinnevi Hospital, Norrkoping for language editing and Dr. Mats Fredrikson in Linkoping Academic Research Centre for statistical advice.
References1. Dahlberg M, Pahlman L, Bergstrom R, Glimelius B. Improved survival in patients with rectal cancer: a population-based register study. Br J Surg 1998;85:515ŌĆō520, PMID: 9607537.
2. Glimelius B, Gronberg H, Jarhult J, Wallgren A, Cavallin-Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003;42:476ŌĆō492, PMID: 14596508.
3. Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638ŌĆō646, PMID: 11547717.
4. Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 2005;23:8697ŌĆō8705, PMID: 16314629.
5. Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol 2005;23:6126ŌĆō6131, PMID: 16135478.
6. Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Late adverse effects of radiation therapy for rectal cancer: a systematic overview. Acta Oncol 2007;46:504ŌĆō516, PMID: 17497318.
7. Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg 2010;97:580ŌĆō587, PMID: 20155787.
8. Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg 2012;99:577ŌĆō583, PMID: 22241246.
9. Glimelius B, Isacsson U, Jung B, Pahlman L. Radiotherapy in addition to radical surgery in rectal cancer: evidence for a dose-response effect favoring preoperative treatment. Int J Radiat Oncol Biol Phys 1997;37:281ŌĆō287, PMID: 9069298.
10. Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys 2009;74:673ŌĆō688, PMID: 19480968.
11. Adell G, Sun XF, Stal O, Klintenberg C, Sjodahl R, Nordenskjold B. p53 status: an indicator for the effect of preoperative radiotherapy of rectal cancer. Radiother Oncol 1999;51:169ŌĆō174, PMID: 10435809.
12. Knutsen A, Adell G, Sun XF. Survivin expression is an independent prognostic factor in rectal cancer patients with and without preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2004;60:149ŌĆō155, PMID: 15337550.
13. Wallin AR, Svanvik J, Adell G, Sun XF. Expression of PRL proteins at invasive margin of rectal cancers in relation to preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:452ŌĆō458, PMID: 16626893.
14. Pfeifer D, Gao J, Adell G, Sun XF. Expression of the p73 protein in rectal cancers with or without preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:1143ŌĆō1148, PMID: 16750334.
15. Loftas P, Onnesjo S, Widegren E, et al. Expression of FXYD-3 is an independent prognostic factor in rectal cancer patients with preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:137ŌĆō142, PMID: 19289258.
16. Bibert S, Roy S, Schaer D, Felley-Bosco E, Geering K. Structural and functional properties of two human FXYD3 (Mat-8) isoforms. J Biol Chem 2006;281:39142ŌĆō39151, PMID: 17077088.
17. Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol 2004;24:97ŌĆō105, PMID: 14654946.
18. Kayed H, Kleeff J, Kolb A, et al. FXYD3 is overexpressed in pancreatic ductal adenocarcinoma and influences pancreatic cancer cell growth. Int J Cancer 2006;118:43ŌĆō54, PMID: 16003754.
19. Syk E, Lenander C, Nilsson PJ, Rubio CA, Glimelius B. Tumour budding correlates with local recurrence of rectal cancer. Colorectal Dis 2011;13:255ŌĆō262, PMID: 19912282.
20. Syk E, Glimelius B, Nilsson PJ. Factors influencing local failure in rectal cancer: analysis of 2315 patients from a population-based series. Dis Colon Rectum 2010;53:744ŌĆō752, PMID: 20389208.
21. Ragnhammar P, Brorsson B, Nygren P, Glimelius B. A prospective study of the use of chemotherapy in Sweden and assessment of the use in relation to scientific evidence. Acta Oncol 2001;40:391ŌĆō411, PMID: 11441943.
22. Meding S, Balluff B, Elsner M, et al. Tissue-based proteomics reveals FXYD3, S100A11 and GSTM3 as novel markers for regional lymph node metastasis in colon cancer. J Pathol 2012;228:459ŌĆō470, PMID: 22430872.
Fig.┬Ā1Immunohistochemistry showing FXYD-3 expression in the primary tumor (H&E, 10├Ś magnification). (A) Weak FXYD-3 expression in the primary tumor. (B) Strong FXYD-3 expression in the primary tumor.
Fig.┬Ā2Time to local recurrence in all 129 patients. Weak FXYD-3 expression (grades 0 + 1 + 2, n = 106) vs. strong (grade 3, n = 23).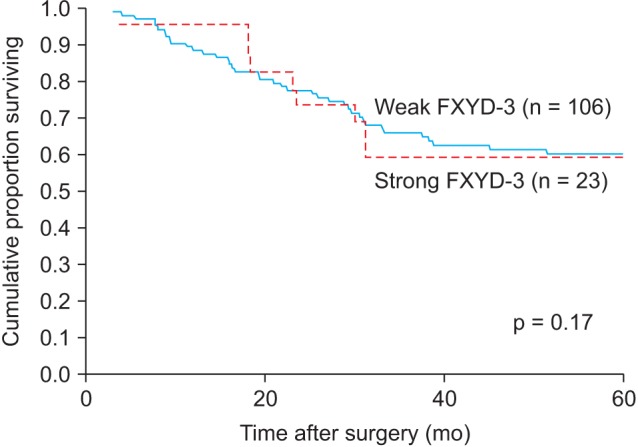
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |