 |
 |
AbstractPurposeThis study aimed to evaluate the long-term survival outcomes and prognostic factors that affect the clinical outcomes of patients who underwent surgery and postoperative radiotherapy for major salivary gland mucoepidermoid carcinoma (MEC).
Materials and MethodsWe retrospectively reviewed the clinical data of 44 patients who underwent surgery followed by radiotherapy for primary MEC of the major salivary glands between 1991 and 2014. The median follow-up period was 9.8 years (range, 0.8 to 23.8 years).
ResultsThe overall outcomes at 5 and 10 years were 81.5% and 78.0% for overall survival (OS), 86.2% and 83.4% for disease-free survival, 90.6% and 87.6% for locoregional recurrence-free survival, and both 90.5% for distant metastasis-free survival (DMFS). Histologic grade was the only independent predictor of OS (low vs. intermediate vs. high; hazard ratio = 3.699; p = 0.041) in multivariate analysis. A poorer survival was observed among patients with high-grade tumors compared with those with non-high-grade tumors (5-year OS, 37.5% vs. 91.7%, p < 0.001; 5-year DMFS, 46.9% vs. 100%, p < 0.001).
IntroductionMucoepidermoid carcinoma (MEC) is a rare malignancy among head and neck cancers; however, it is the most common type of salivary gland malignancy and accounts for 10% of all types of tumors, including benign and 30%-35% of malignant tumors [1,2]. Approximately 60% of MECs originate from the major salivary glands, and the parotid gland is the predominant site [3-5]. These neoplasms exhibit various clinical courses from indolent to highly aggressive locally and highly metastatic. Surgery has been the principal treatment modality for salivary gland MEC, and postoperative radiotherapy has recently been used for T3–4 tumors, neck node metastases, close or positive resection margins, and high-grade tumors. However, there is limited information on the clinicopathologic prognostic factors of patients who underwent surgery and postoperative radiotherapy [6-10]. Hence, to investigate treatment outcomes, we retrospectively reviewed patients with major salivary gland MEC who underwent surgery and postoperative radiotherapy, which included local tumor control, survival, and prognostic factors.
Materials and Methods1. Patient characteristicsA total of 45 patients with primary MEC of the major salivary glands underwent surgical resection and postoperative radiotherapy between 1991 and 2014 at Asan Medical Center. After a review of the pathologic findings, 44 patients were enrolled, and 1 patient with neuroendocrine carcinoma was excluded. We evaluated clinicopathologic variables such as the age, gender, symptoms, disease location, tumor size, T stage, and N stage of the patients. All patients underwent disease staging according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. A histopathologic review was re-performed by a head and neck pathologist who reviewed the histologic features of all patients with the available microscopic slides and paraffin-embedded tissue blocks. Histologic grading was performed according to the Armed Forces Institute of Pathology (AFIP) criteria proposed by Goode et al. [5].
2. Radiotherapy and follow-upIn general, postoperative radiotherapy was performed for stage T3–4 tumors, positive resection margin, perineural invasion, positive neck node, or high-grade tumor. The target volume for tumors without lymph node involvement was the surgical bed. For high-grade tumors and tumors with lymph node involvement, the target volume included the surgical bed, involved nodal stations, and ipsilateral neck nodes at level I–IV. In general, a dose of 60 Gy was delivered to the surgical bed via conventional fractionation with photons, electrons, or both using a linear accelerator. No patient received adjuvant chemotherapy.
Patients were followed up 4–6 weeks following the completion of therapy and then every 3 months for the first 2 years. Subsequently, the patients were monitored every 6 months. A physical examination at each follow-up visit and a CT scan of the head and neck were performed as needed.
3. Statistical analysisThe baseline follow-up date was the day of surgery, and the last follow-up date was the last hospital visit or phone call date. The overall survival (OS) was calculated from the baseline date to the date of the patient’s death, censoring the last follow-up date. The locoregional recurrence-free survival (LRFS) and the distant metastasis-free survival (DMFS) were calculated from the baseline date to the first recurrence date, censoring death or the last follow-up date. Survival curves were produced using the Kaplan-Meier method and the log-rank test. All statistical analyses were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA).
Results1. Patient characteristicsThe median patient age was 46 years (range, 12 to 75 years). At the time of diagnosis, all patients were presented with a palpable mass and median symptom duration of 5 months (range, 1 to 240 months). MEC was found in the parotid gland of 38 patients (86.4%), in the submandibular gland of 4 patients (9.1%), and in the sublingual gland of 2 patients (4.5%). In addition, 28 patients (63.6%) had a confirmed AJCC staging group of more than stage III. Among 38 patients with parotid MEC, total parotidectomy was most commonly performed (n = 31). In cases of submandibular or sublingual MEC, surgery with a wide excision was performed for 5 patients, and mass excision was performed for 1 patient. Furthermore, neck dissection was performed for 19 patients (43.2%); 16 of them underwent selective neck dissection, and 3 of them underwent modified radical neck dissection. The median number of positive nodes was 2 (range, 1 to 33) in cases with node involvement. The median radiotherapy dose was 59.7 Gy (range, 39.6 to 66.6 Gy). Three-dimensional planning or intensity-modulated radiotherapy was performed for 24 patients (54.6%) (Table1).
The histopathologic characteristics are shown in Table 2. An extraglandular extension was observed in 31 patients (70.5%), and 24 patients (54.5%) had positive margins. According to the AFIP system, low-grade, intermediate-grade, and high-grade tumors were detected in 16, 20, and 8 patients, respectively.
2. Treatment outcome and survivalThe median follow-up duration was 9.8 years. At the time of analysis, 7 patients (15.9%) had recurrences (locoregional failures in 3 patients, distant failures in 2 patients, and both locoregional and distant failures in 2 patients) (Table 3). The average time interval from the initial treatment to local recurrence or distant metastasis was 21.4 months (range, 5 to 63 months). The sites of distant metastases included the lungs (n = 3) and bone (n = 1). Salvage surgery was performed on 3 patients with locoregional recurrence, and 2 of them were alive with a ‘no evidence of disease’ status at their last follow-up. The 5-year and 10-year LRFS, DMFS, and disease-free survival (DFS) rates were 90.6% and 87.6%, both 90.5%, and 86.2% and 83.4%, respectively. The 5-year and 10-year OS rates were 81.5% and 78.0%, with 11 patients dying during the follow-up period. Among the patients, 5 died due to MEC; 4 of them died from distant metastasis, and 1 of them died from locoregional recurrence. Therefore, the 5-year cause-specific survival was 87.9%.
3. Prognostic factorsUnivariate analysis revealed that tumor location (parotid vs. non-parotid, p = 0.031), N stage (N0–1 vs. N2, p = 0.018), and lymphovascular invasion (p = 0.005) were significant prognostic factors of LRFS. Nodal stage, histologic grade, and lymphovascular invasion showed prognostic significance for DMFS, DFS, and OS (Table 4). In addition to these factors, tumor location and extraglandular extension were significant prognostic factors for OS. Multivariate analysis revealed that histologic grade (hazard ratio = 3.699; 95% confidence interval, 1.057–12.941; p = 0.041) was an independent prognostic factor for OS (Fig. 1). The 5-year OS and DMFS rates were 37.5% and 46.7% for patients with high-grade tumors and 91.7% and 100% for those with non-high-grade tumors (p < 0.001) (Fig. 2A, 2B). Out of 8 patients with high-grade tumors, 4 patients died from their disease; 2 died due to distant metastasis, and 2 died due to local recurrence with distant failures.
Discussion and ConclusionSeveral retrospective studies of salivary gland malignancies have established the positive role of postoperative radiotherapy in the treatment of patients with poor prognostic factors [11-14]. Most of the studies included patients with heterogeneous histologic subtypes. However, some studies considered histologic type as a prognostic factor for survival [12,15-17]. There is limited information on the clinical outcomes of patients with salivary gland MEC who underwent surgery and postoperative radiotherapy [6,8-10,18,19]. Ghosh-Laskar et al. [18] reported 5-year DFS and OS rates of 77.8% and 92.4% among 113 patients with parotid gland MEC, 61% of whom received adjuvant radiotherapy. Guzzo et al. [6] reported a 5-year DFS rate of 48.8% for 35 cases of head and neck MEC treated with postoperative radiotherapy. This result was relatively poor because most of the patients who received adjuvant radiotherapy had advanced locoregional disease, high-grade tumors, and tumors with positive resection margins. Chen et al. [10] assessed the clinical outcomes of patients who underwent surgery and postoperative radiotherapy for MEC of the parotid gland. In that study, the 5-year OS rate was 79%, which is comparable with the result of our study (5-year OS, 81.5%).
The clinical stage and histologic grade of MEC have been reported to be important prognostic factors that affect therapeutic decision-making [6,9,10,18-20]. In our study, the histologic grade was an independent prognostic factor for OS in multivariate analysis. There was a significant worsening of OS and DMFS from low-grade to intermediate-grade to highgrade MEC. The 5-year OS rate was 37.5% among patients with high-grade tumors compared with 91.7% among those with non-high-grade tumors. Our results are in agreement with the results of other studies showing the favorable prognosis of low-grade and intermediate-grade MEC [9,10,20]. Chen et al. [10] reported a 5-year OS rate of 83.3% among patients with non-high-grade tumors compared with 52% among those with high-grade tumors. Nance et al. [9] revealed that survival outcomes were similar among patients with low-grade and intermediate-grade MEC of the head and neck. In another study by Aro et al. [19], the survival outcomes of patients with intermediate-grade MEC were found to be similar to those of patients with high-grade MEC. However, the number of patients in the intermediate-grade MEC group (7 of 52 patients) was relatively small in that study. The prognosis of intermediate-grade MEC remains controversial.
Different sets of histologic criteria have been used for grading in previous studies. Therefore, the survival outcomes observed in retrospective studies should be interpreted with caution. There are three predominant grading systems, including the Batsakis and Luna modification of the Healey system [21], the Brandwein system [22], and the AFIP system [5]. Brandwein et al. [22] found that the AFIP grading criteria tended to downgrade MEC. Therefore, they proposed a modified grading system with the addition of other criteria such as vascular invasion and tumor infiltration pattern. Luna [4] compared all three grading systems in 2006 and found that the Batsakis and Luna modification of the Healey system and the Brandwein system were more accurate for grading and that the AFIP system tended to downgrade MEC. However, as the AFIP system is easier to reproduce, we prospectively graded the tumors based on this system [5] (Table 5). Despite the limitation, this is one of the few studies that have reported the survival outcomes of patients who underwent surgery and postoperative radiotherapy for MEC based on histologic grade and that have stated the grading system used for reevaluating the grade [10]. Institutions should use a uniform grading system for MEC to accurately assess the prognosis and set treatment guidelines.
Our data demonstrated a high rate of locoregional control, whereas patients with high-risk features such as high-grade or advanced stage showed poor OS and DMFS. For squamous cell carcinomas originating from other sites in the head and neck, postoperative concurrent chemotherapy, particularly with cisplatin, has been shown to improve OS [23,24]. However, as far as we know, there has been no prospective study of the role of concurrent chemotherapy in salivary gland cancer. Several retrospective studies have been reported, but most are small series with a variety of histology and various chemotherapy regimens [25-28]. In a series of 24 cases from Pederson et al. [26], concurrent postoperative chemotherapy (paclitaxel, 5-fluorouracil, hydroxyurea) with radiotherapy for locoregionally advanced and high-risk salivary gland malignancies showed 5-year OS rate of 59%. Mifsud et al. [27] analyzed 140 patients with high-risk salivary gland carcinomas treated with postoperative concurrent chemoradiotherapy (37 patients) or radiotherapy (103 patients). In their population, concurrent platinum-based chemotherapy did not significantly improve progression-free survival compared to radiotherapy alone. The use of concurrent chemotherapy in the treatment of high-risk salivary gland cancer is not recommended until prospective evidence is available.
In our current study, the parotid gland was the predominant site, followed by the submandibular gland and the sublingual gland; these results are in agreement with other published findings [5,12,20]. Our result demonstrated that patients with parotid gland tumors had a better prognosis than those with non-parotid tumors; however, this result showed no significance in multivariate analysis. Spiro et al. [29] found that metastases from the submandibular gland were more frequent than those from the other major salivary glands. Some studies have indicated that patients with submandibular gland tumors should receive more intensive treatment [5,30]. In a recent study by Granic et al. [31], submandibular/sublingual MECs contributed to a poor prognosis compared with parotid or other minor salivary gland MECs. However, Brandwein et al. [22] reported that there was no correlation between the tumor site and prognosis. They indicated that the reported poor survival outcomes of patients with submandibular gland MEC in the literature may be associated with a lesser cuff of the surrounding normal tissue of submandibular gland resection specimen compared with that of a superficial parotidectomy specimen.
In conclusion, this study found that histologic grade was the most important prognostic factor in cases of major salivary MEC treated with surgery and postoperative radiotherapy. Although surgery followed by radiotherapy could result in excellent locoregional control, the poor prognosis of high-grade tumors emphasizes the need for a more aggressive treatment approaches such as adjuvant chemotherapy or targeted therapy.
Fig. 2.Comparison of (A) overall survival and (B) distant metastasis-free survival rates for patients with high-grade versus non-highgrade tumors. 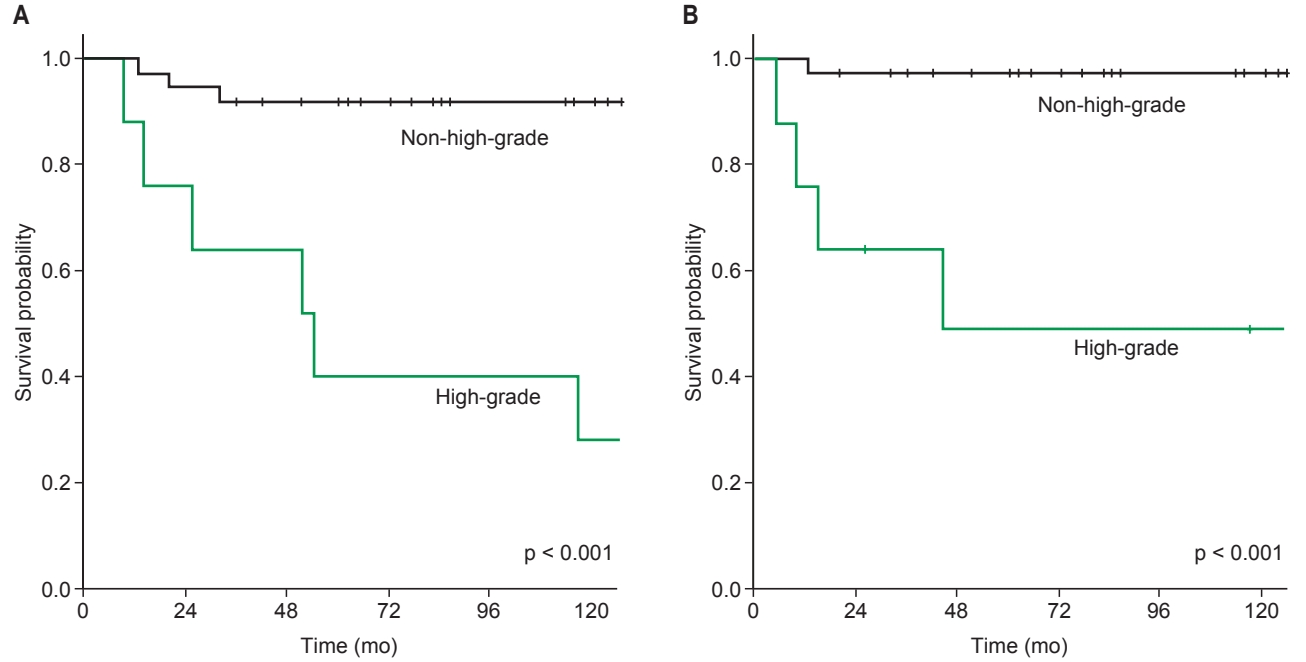
Table 1.Clinical characteristics of the enrolled patients with major salivary gland MEC who underwent postoperative radiotherapy (n = 44) Table 2.Histopathologic characteristics of the enrolled patients with major salivary gland MEC who underwent postoperative radiotherapy (n = 44) Table 3.The summary of recurrent cases (n = 7) Table 4.Univariate analysis of prognostic factors for survival of patients with major salivary gland MEC (n = 44) References1. Eveson JW, Cawson RA. Salivary gland tumours: a review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol 1985;146:51–8.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177–84.
5. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998;82:1217–24.
6. Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol 2002;9:688–95.
7. Kokemueller H, Brueggemann N, Swennen G, Eckardt A. Mucoepidermoid carcinoma of the salivary glands: clinical review of 42 cases. Oral Oncol 2005;41:3–10.
8. Rapidis AD, Givalos N, Gakiopoulou H, et al. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol 2007;43:130–6.
9. Nance MA, Seethala RR, Wang Y, et al. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 2008;113:2082–9.
10. Chen AM, Lau VH, Farwell DG, Luu Q, Donald PJ. Mucoepidermoid carcinoma of the parotid gland treated by surgery and postoperative radiation therapy: clinicopathologic correlates of outcome. Laryngoscope 2013;123:3049–55.
11. Garden AS, el-Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys 1997;37:79–85.
12. Terhaard CH, Lubsen H, Van der Tweel I, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2004;26:681–93.
13. Kaur J, Goyal S, Muzumder S, Bhasker S, Mohanti BK, Rath GK. Outcome of surgery and post-operative radiotherapy for major salivary gland carcinoma: ten year experience from a single institute. Asian Pac J Cancer Prev 2014;15:8259–63.
14. North CA, Lee DJ, Piantadosi S, Zahurak M, Johns ME. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys 1990;18:1319–26.
15. Theriault C, Fitzpatrick PJ. Malignant parotid tumors: prognostic factors and optimum treatment. Am J Clin Oncol 1986;9:510–6.
16. Renehan A, Gleave EN, Hancock BD, Smith P, McGurk M. Long-term follow-up of over 1000 patients with salivary gland tumours treated in a single centre. Br J Surg 1996;83:1750–4.
17. Lopes MA, Santos GC, Kowalski LP. Multivariate survival analysis of 128 cases of oral cavity minor salivary gland carcinomas. Head Neck 1998;20:699–706.
18. Ghosh-Laskar S, Murthy V, Wadasadawala T, et al. Mucoepidermoid carcinoma of the parotid gland: factors affecting outcome. Head Neck 2011;33:497–503.
19. Aro K, Leivo I, Makitie AA. Management and outcome of patients with mucoepidermoid carcinoma of major salivary gland origin: a single institution's 30-year experience. Laryngoscope 2008;118:258–62.
20. Pires FR, de Almeida OP, de Araujo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg 2004;130:174–80.
21. Batsakis JG, Luna MA. Histopathologic grading of salivary gland neoplasms. I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol 1990;99(10 Pt 1):835–8.
22. Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25:835–45.
23. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52.
24. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44.
25. Tanvetyanon T, Qin D, Padhya T, et al. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch Otolaryngol Head Neck Surg 2009;135:687–92.
26. Pederson AW, Salama JK, Haraf DJ, et al. Adjuvant chemoradiotherapy for locoregionally advanced and high-risk salivary gland malignancies. Head Neck Oncol 2011;3:31.
27. Mifsud MJ, Tanvetyanon T, Mccaffrey JC, et al. Adjuvant radiotherapy versus concurrent chemoradiotherapy for the management of high-risk salivary gland carcinomas. Head Neck 2016;38:1628–33.
28. Gebhardt BJ, Ohr JP, Ferris RL, et al. Concurrent chemoradiotherapy in the adjuvant treatment of high-risk primary salivary gland malignancies. Am J Clin Oncol 2018;41:888–93.
29. Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg 1978;136:461–8.
|
|
|||||||||||||||||||||||||||||||||||||||||

 |
 |