 |
 |
AbstractPurposeThe indication of elective neck treatment (ENT) for clinically N0 (cN0) paranasal sinus (PNS) carcinoma remains unclear. We aimed to investigate different treatment outcomes regarding ENT and propose optimal recommendations for ENT.
Materials and MethodsWe identified patients with cN0 PNS carcinoma who underwent curative-intent treatment between 1992 and 2015. Survival outcomes and pattern of failure were compared between patients who received ENT and those who did not. We sought to identify significant patient or pathologic factors regarding treatment outcomes.
ResultsAmong 124 patients meeting the inclusion criteria, 40 (32%) received ENT (‘ENT (+) group’) and 84 (68%) did not (‘ENT (−) group’). With a median follow-up of 54 months, the 5-year overall survival (OS) was 67%, and the 5-year progression-free survival (PFS) was 45%. There was no significant difference between the ENT (+) and ENT (−) groups regarding OS (p = 0.67) and PFS (p = 0.50). Neither group showed a significantly different pattern of failure, including regional failure (p = 0.91). There was no specific benefit, even in the subgroups analysis by tumor site, histologic type, and T stage. Nevertheless, patients who ever had regional and/or distant failure showed significantly worse prognosis.
ConclusionENT did not significantly affect the survival outcome or pattern of failure in patients with cN0 PNS carcinomas, showing that ENT should not be generalized in this group. However, further discussion on the optimal strategy for ENT should continue because of the non-negligible regional failure rates and significantly worse prognosis after regional failure events.
IntroductionParanasal sinus (PNS) malignant neoplasms are a rare form of head and neck malignancy, accounting for <3% of upper aerodigestive tract malignancies [1], with approximately 0.6 cases per 100,000 people in the United States [2]. PNS cancers usually present as locally advanced disease due to the late onset of symptoms or the fact that early symptoms resemble common sinusitis or rhinitis. As a result, the treatment strategy is focused on achieving adequate local control while preserving critical nearby anatomic structures [3]. To our knowledge, although there are no randomized trials comparing different treatment strategies, surgery followed by adjuvant radiation is generally accepted as the mainstay of treatment, when resectable [3-5].
PNS malignancies have a relatively low incidence of regional nodal metastases at initial diagnosis, ranging from 8.3% to 21.9% [6-8]. This low incidence could be explained by a relatively sparse network of lymphatic drainage in the maxillary sinus, which consists most of the PNS malignancies. This low incidence has led to controversies regarding treatment of the neck in patients with clinically node-negative (cN0) PNS cancer. Some researchers argue that elective neck treatment (ENT) in these patients is unnecessary as no factors predictive of regional nodal metastasis are found, making the patient selection for ENT difficult [9]. In addition, the absolute regional nodal metastasis rate remains relatively low and is usually accompanied by local failure, which remains a major problem that results in poor prognosis [10]. On the other hand, several other retrospective reports insist that ENT is beneficial, especially when some adverse features exist [11,12]. Recently, a retrospective review of regional failure (RF) in cN0 patients with squamous cell carcinoma of the maxillary sinus with treatment of primary site only, showed that the rate of occult neck metastases was not negligible (14.3%) [13]. As there have been no randomized trials regarding this issue, the controversy over ENT is ongoing.
Despite considerable debate surrounding this issue, little is known about the need for ENT or pattern of failure in PNS cancers other than cancer of the maxillary sinus. In light of these issues and the ongoing controversy, we conducted a retrospective study to investigate different treatment outcomes between patients with and without ENT in cN0 PNS carcinoma, including sites other than the maxillary sinus and pathology other than squamous cell carcinoma. In this study, we aimed to propose optimal indications regarding ENT in patients with cN0 PNS cancers and investigate the prognosis of patients with RF.
Materials and Methods1. Patient selectionWe searched the institutional database for all patients who were diagnosed with PNS cancer at Yonsei Cancer Center from January 2000 to December 2015. Among 304 patients who were identified, those who were initially diagnosed as cN0 and who received any form of curative-intent treatment with a sufficient follow-up period at our institution were eligible. Patients with initially node-positive PNS cancers were excluded (n = 35); those who had a pathologic diagnosis of melanoma, olfactory neuroblastoma, sarcoma, or hematology malignancy were also excluded (n = 40). Next, we excluded patients who did not have complete medical records or did not receive a full course of the initial intended therapy (n = 28). Finally, we identified a total of 124 patients diagnosed with cN0 PNS cancer who met the eligibility criteria.
2. Data collectionDemographic, histopathologic, and treatment characteristics of individual patients were collected from the medical records, including age at diagnosis, sex, primary tumor site, histologic type, T stage, tumor size, and initial treatment type. All patients had an initial evaluation based on either computed tomography (CT) or magnetic resonance imaging (MRI). T stage was reassessed according to the 7th edition of the American Joint Committee on Cancer TNM classification. Patients who did not undergo surgery had clinical staging based on CT or MRI. Those who underwent surgery had pathologic staging based on pathology reports. The initial treatment type included information about chemotherapy, surgery, radiotherapy, and whether ENT was performed. When surgery was performed, surgical pathology characteristics—e.g., status of the resection margin (RM), perineural invasion (PNI), and lymphovascular invasion (LVI)—were also collected if such reports existed.
Patients who received ENT were defined as those who underwent elective neck dissection and/or elective neck irradiation. The irradiated dose of the elective neck nodal chain was often 45–54 Gy in conventional fractions. At our institution, ENT was performed based on the clinician’s decision regarding risk factors of nodal metastasis (e.g., advanced T stage, histologic subtype, LVI) in each patient.
The main interest of this study was the impact of ENT on overall survival (OS), progression-free survival (PFS), and pattern of failure. For this purpose, we reviewed the pattern of failure and time to relapse of patients. Data of the initial failure site, time to relapse, and specific salvage treatment after failure were collected. Cumulative failure events in each patient were also collected. Failure patterns were classified as local, regional, or distant failure (DF). Local failure was defined as any failure occurring in structures near the primary tumor site. RF was defined as any failure occurring either in the neck nodal chains or retropharyngeal space. For RF, the nodal level of the failure site was specified according to an established consensus guideline [14]. All other failures were categorized as DFs.
3. Statistical analysisThe characteristics of patients who received ENT and those who did not receive ENT were compared via the Pearson χ2 test and independent t-test. OS was defined as the time from pathologic diagnosis to death from any cause. PFS was defined as the time from pathologic diagnosis to any form of disease progression or death. If no events had occurred, the patient was censored at the last follow-up date. OS and PFS were analyzed according to different prognostic factors using the Kaplan-Meier method. Any demographic, pathologic, and treatment characteristics relevant to survival outcome were investigated using the log-rank test for univariate analysis and Cox proportional hazards regression for multivariate analysis. We also divided the total patients into subgroups according to different tumor sites and histologic subtypes, to determine if any subgroups had a different outcome. The OS and PFS of patients who had regional or DF were compared with patients who did not, to identify differences in survival outcomes. Total deaths, total failures, local failures, RFs, and DFs between the ENT (+) and ENT (−) groups were compared using the Pearson χ2 test. In terms of RF, each nodal recurrence site in patients with RF was specified, and common sites of RF were identified. Regarding statistical analysis, two-sided significance tests were performed, and statistical significance was set at p-value ≤0.05. Statistical analysis was conducted using IBM SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
Results1. Patient characteristicsAs shown in Fig. 1, among 124 patients, 40 (32%) received ENT and 84 (68%) did not receive ENT. In the ENT (+) group, 35 (87.5%) had elective neck irradiation, 4 (10%) had elective neck dissection, and 1 patient (2.5%) had both. The baseline demographic, histopathologic, and treatment characteristics of the ENT (+) and ENT (−) groups are listed in Table 1. The median follow-up period was 36 months (range, 2 to 268 months) and 64 months (range, 3 to 288 months) for the ENT (+) and ENT (−) groups, respectively. Overall, most factors were not significantly different between the two groups. The most common site of PNS cancer was the maxillary sinus in both groups—37 of 40 (92%) in the ENT (+) vs. 72 of 84 (86%) in the ENT (−) group, followed by the ethmoid sinus and sphenoid sinus; we found no patients with frontal sinus cancer who were eligible for this study. The most common histologic type was squamous cell carcinoma—29 of 40 (73%) in the ENT (+) vs. 53 of 84 (63%) in the ENT (−) group. No significant difference in T stage, tumor size, or pathologic factors, including RM, LVI, or PNI, between the groups was noted. In terms of treatment modalities, the treatment scheme for all patients was classified into five major categories: neoadjuvant radiotherapy (±chemotherapy) + operation (Op), neoadjuvant chemotherapy + Op, Op ± adjuvant chemotherapy, Op + adjuvant radiotherapy (±chemotherapy), or definitive radiotherapy (±chemotherapy). Chemotherapy was performed more often in the ENT (+) group (21 of 40; 52%) than in the ENT (−) group (23 of 84; 27%) Radiotherapy for the primary mass was also performed more often in the ENT (+) than in the ENT (−) group. A difference in radiotherapy modality was noted, with intensity-modulated radiotherapy used more frequently in the ENT (+) group (35 of 40; 88%) than in the ENT (−) group (18 of 84; 21%).
2. Treatment outcomes and prognostic factorsAfter a median follow-up period of 54 months, a total of 47 deaths and 75 failures occurred in all patients. The 3-year and 5-year OS rates were 74% and 67%, respectively, in the total patients; the 3-year and 5-year PFS rates were 55% and 45%, respectively (Fig. 2). In the total patients, local failure occurred in 52 patients (42%), RF occurred in 21 patients (17%), and DF occurred in 34 patients (27%). We also investigated whether any significant demographic, histopathologic, or treatment factors showed prognostic values regarding OS and PFS. On univariate analysis of OS, diagnosis of the primary site (categorized as ethmoid sinus vs. other) was the only significant factor (p < 0.01). RM (categorized as negative vs. close or positive margin, p = 0.12) and the addition of chemotherapy (p = 0.08) showed marginal significance. After multivariate analysis concerning the above factors with elective neck treatment, no factors showed significant prognostic values. Univariate analysis of PFS showed similar results. Diagnosis of the primary site (p = 0.05) and RM (p = 0.02) were significant factors for PFS, and the addition of chemotherapy (p = 0.06) showed marginal significance. On multivariate analysis of PFS with the abovementioned factors, RM was the only significant factor (p = 0.02) (Table 2).
3. Impact of ENT on survival and pattern of failureRegarding OS, the addition of ENT did not show statistically significant improvement (p = 0.67). The 3-year and 5-year OS rates were 72% and 64% in the ENT (+) group and 74% and 69% in the ENT (−) group, respectively (Fig. 3A). The addition of ENT also did not show significant improvement in PFS (p = 0.50). The 3-year and 5-year PFS rates were 59% and 33% in the ENT (+) group, and 54% and 48% in the ENT (−) group, respectively (Fig. 3B).
When the ENT (+) and ENT (−) groups were compared according to different failure patterns, no statistically significant difference was noted between the two groups, as shown in Table 3. RF occurred in 7 of 40 (18%) patients in the ENT (+) group, and 14 of 84 (17%) patients in the ENT (−) group (p = 0.91). Details of patients who had RFs without ENT are specified in Table 4. Among these patients, the most common site of the primary tumor was the maxillary sinus (10 of 14; 71%), followed by the ethmoid sinus and sphenoid sinus. The most frequent histologic subtype was squamous cell carcinoma (9 of 14; 64%). RF was most commonly noted in ipsilateral level I or II, in 10 of 14 patients. The RF patterns of this group are schematized in Fig. 4A and 4B. In the ENT (+) group, 4 of 7 (57%) RFs were in-field failures after elective neck irradiation, 1 of 7 (14%) RF was failure in the electively dissected neck, while 2 of 7 (29%) RFs were out-field failures in the contralateral neck after elective neck irradiation. There was no marginal failure. Radiation dose of in-field failures were 44, 46, 48, and 51 Gy for each failures.
4. Survival outcomes in patients with DF and/or RFWe investigated survival outcomes with respect to DFs and/or RFs. Between patients who had DFs (‘DF (+) group’, n = 34) and those who did not (‘DF (−) group’, n = 90), significantly worse survival outcome in all survival endpoints was noted in the failure-positive group (Fig. 5A). The 5-year OS and PFS rates were 51% and 23% in the DF (+) group and 74% and 55% in the DF (−) group, respectively (p < 0.01). Among patients who had RFs (‘RF (+) group’, n=21) and those who did not (‘RF (−) group’, n = 103), we found similar results when OS and PFS were compared between the two groups (Fig. 5B). The 5-year OS and PFS rates were 41% and 14%, respectively, in the RF (+) group, in contrast to 74% and 52%, respectively, in the RF (−) group (p < 0.01). In the RF (+) group, 13 of 21 patients (62%) had local failure preceding RF, and a total of 14 deaths occurred. Of these 14 deaths, 6 were due to sequential distant metastasis causing organ failure, such as of the lung and liver. Five deaths were owing to local progression combined with regional metastasis leading to invasion of critical organs, such as the brain and spinal cord.
5. Subgroup analysis of OS and PFS by tumor site, histologic type, and T stageWe performed subgroup analysis of OS and PFS by tumor site, histologic type, and T stage. Table 5 summarizes the impact of ENT on OS and PFS in each subgroup. Of the 109 patients with maxillary sinus cancer, 41 (38%) were T4 stage. Regarding pathologic characteristics for which specific information was available, 71% (47 of 69) of patients had close or positive RM, 41% (9 of 22) were PNI positive, and 22% (4 of 18) were LVI positive. In this subgroup, 37 patients (34%) received ENT. The 5-year OS and PFS rates were 67% and 33% in the ENT (+) group and 76% and 53% in the ENT (−) group, respectively (all p > 0.05) (Fig. 6). The RF rates among these patients were 81% in the ENT (+) group and 86% in the ENT (−) group (p = 0.49). Among 15 patients with ethmoid sinus or sphenoid sinus cancer, 3 patients (20%) received ENT. The 5-year OS rates—33% in the ENT (+) vs. 28% in the ENT (−) group—and PFS rates—33% in the ENT (+) vs. 18% in the ENT (−) group—were also not improved by ENT (all p > 0.05). The RF rates in this group were 0% in the ENT (+) group and 33% in the ENT (−) group (p = 0.24).
Among 82 patients with squamous cell carcinoma, 41 (50%) had T4 stage disease. For patients in whom specific pathologic characteristics were available, 61% (25 of 41) had close or positive RM, 0% (0 of 11) was PNI positive, and 9% (1 of 11) were LVI positive. In this subgroup, 29 patients (35%) received ENT. The 5-year OS and PFS rates were 61% and 39% in the ENT (+) group and 63% and 44% in the ENT (−) group, respectively (all p > 0.05) (Fig. 7). The RF rates in this group were 86% in the ENT (+) group and 83% in the ENT (−) group (p = 0.71). In 23 patients with adenoid cystic carcinoma, 15 (35%) patients received ENT. 5-year OS rate was not improved by ENT—63% in the ENT (+) vs. 86% in the ENT (−) group, p = 0.15, while 5-year PFS rate showed statistically significant difference—0% in the ENT (+) vs. 65% in the ENT (−) group, p = 0.03. No significant difference in RF rates were noted according to ENT—25% in the ENT (+) vs. 20% in the ENT (−) group, p = 0.78. Among 5 patients with adenocarcinoma, all patients didn’t receive ENT. The 5-year OS and PFS rates were 75% and 53%, respectively. No patient had regional recurrence in this subgroup. In subgroup analysis excluding adenoid cystic carcinoma from the total patient group, no different outcomes or significant improvement in survival by ENT was noted.
Of the 71 patients staged as T2 or T3, 44 (62%) had squamous cell carcinoma. For patients in whom specific pathologic characteristics were available, 69% (34 of 49) had close or positive RM, 39% (5 of 13) were PNI positive, and 27% (3 of 11) were LVI positive. In this subgroup, 19 patients (27%) received ENT. The 5-year OS rates showed no statistically significant by ENT—61% in the ENT (+) vs. 78% in the ENT (−) group, p = 0.41, while 5-year PFS rates showed significant difference—25% in the ENT (+) vs. 54% in the ENT (−) group, p = 0.04. The RF rates in this group was 32% in the ENT (+) group and 13% in the ENT (−) group (p = 0.08). Among 53 patients with T4 stage, 21 patients (40%) received ENT. The 5-year OS and PFS rates were 67% and 40% in the ENT (+) group and 52% and 38% in the ENT (−) group (all p > 0.05). The RF rates in this group was 5% in the ENT (+) group and 22% in the ENT (−) group (p = 0.09).
Discussion and ConclusionIn the present study, we showed that ENT has no benefit regarding survival rates or failure patterns, including RF rates, in patients with cN0 PNS cancer. RM was the only significant factor for survival rate; however, the addition of ENT did not have a significant impact on OS, PFS, or pattern of failure. This result was consistent in the subgroup analysis by tumor site, histologic type, and T stage. However, the RF (+) group showed dismal prognosis, similar to that of the DF (+) group, mainly due to sequential distant metastasis or combined local progression, suggesting the need for further investigation of proper regional management.
The efficacy of ENT in PNS cancer has been a controversial issue for many years. As a result, there has been no ‘gold standard’ regarding ENT in patients with cN0 PNS cancer. Our results, showing no significant benefits of ENT, are consistent with those of several studies stating that ENT is unnecessary in patients with cN0 PNS cancer. The clinical significance of ENT has been questioned by several researchers owing to the relatively low incidence of RF compared with local failure. Kim et al. [10] analyzed 116 patients with maxillary sinus squamous cell carcinoma. During follow-up, the RF rate (19%) was far lower than the local failure rate (68%). Park et al. [15] also recently reported that the rate of occult cervical metastasis in maxillary squamous cell carcinoma was not sufficiently high (15%) to routinely recommend elective neck dissection. The RF rates in previous studies are fairly consistent with the RF rate in our study (17%). Moreover, previous researchers have pointed out that no subgroup of patients at higher risk of RF has been identified [9]. Our findings, showing no benefit of ENT in the subgroup analysis by tumor site, histologic type and T stage, support this idea. In addition to these findings, our study showed that ENT had no benefit with respect to OS, PFS, or RF rates. Also, pattern of failure revealed that RF mostly occurred in maxillary sinus cancer or squamous cell carcinoma when ENT was not performed. The most common RF site was ipsilateral neck level I or II. We believe that these findings could provide useful information to radiation oncologists when they need to decide whether to perform elective neck irradiation or not in cN0 PNS cancer patients. In addition, to our knowledge, this study is one of the few studies including a considerable number of patients that directly compares the efficacy of ENT with respect to survival and pattern of failure in patients with cN0 PNS cancer.
However, our results contradict some previous arguments made in former studies. Jiang et al. [12] and Joosten et al. [13] claimed that elective neck irradiation should be considered in patients with squamous cell and undifferentiated carcinoma or advanced T stage because the RF rate was high (19%–38%) in these patients. Recently, Jeon et al. [16] reported that ENT was effective in preventing RF in squamous cell and undifferentiated carcinoma of the maxillary sinus with posterior wall invasion. Among 8 patients who received ENT in their study, only 1 patient had RF, whereas 9 of 47 patients who received ENT and were initially cN0 experienced RF in our study, showing that ENT might have a considerable effect on survival.
The reason that the results of our study conflict with those of other studies could be explained in several ways. First, the patient subset in our study differs considerably from that of other studies [11-13,16,17]. We enrolled all patients with PNS cancer, including those with ethmoid sinus and sphenoid sinus cancers. We also included histologic subtypes other than squamous cell carcinoma, to determine the efficacy of ENT in all PNS cancers. Although our subgroup analysis by tumor site, histologic type, and T stage did not show positive impact of ENT on OS and PFS, the number of patients in each subgroup might have been insufficient to achieve statistically significant difference since other studies showed clinical benefit in specific patient subsets as aforementioned. Also, the possibility that other factors such as surgical pathology characteristics (e.g., RM, LVI, PNI) could affect survival outcomes cannot be excluded [13,18]. Second, previous studies [12,17] might have included patients that would currently be diagnosed as clinically node positive due to advancements in imaging techniques. Studies conducted before the widespread use of CT or MRI relied on clinical nodal staging based on physical examination or plain radiography, which likely missed some patients that would have been diagnosed as node positive using modern imaging. On the other hand, at our institution, CT has routinely been used for initial staging in head and neck cancer patients, and MRI was added to the routine staging process after the mid-2000s. In other words, our results should be accepted on the basis of precise radiologic evaluation. Lastly, the proportion of patients who underwent chemotherapy differed from that of other groups. In our study, 35% of the total patients had chemotherapy. This differs substantially from other studies specifying the proportion of patients that received chemotherapy (58%–81%) [16,19]. Although our study did not show a significant effect of chemotherapy on survival, we should nevertheless consider the impact of chemotherapy, as some reports insist that survival or RF rates could be affected by chemotherapy [16,19,20].
In our study, univariate analysis of PFS showed that primary site and RM were significant factors whereas chemotherapy showed marginal significance. After multivariate analysis with ENT and the above factors, RM was the only significant factor (p = 0.02). Regarding OS, although RM did show prognostic marginal significance in univariate analysis (p = 0.12), no factors showed significant prognostic values in multivariate analysis. These findings are in line with previous findings that complete surgical resection of the primary tumor is essential for long-term survival in this type of cancer [3,10,19,21]. Local control is the key treatment factor in patients with PNS cancer because of the critical organs located nearby, such as the brain, brainstem, optic nerve, inner ear, and so forth. Repetitive local failures may unfavorably affect patient quality of life and may make local control very difficult. Furthermore, according to a report by Kim et al. [10], local recurrence precedes most nodal failures, again highlighting the importance of local control. Similarly, in our study, 13 of 21 (62%) patients with RF had local failure preceding RF. Especially in the ENT (+) group, all in-field failures had local failure preceding RF, or simultaneously with RF. These local failures might have contributed to the relatively high in-field failure rate despite that adequate elective irradiation dose of 44-51 Gy was given. Moreover, patients who underwent chemotherapy had worse prognosis in univariate analysis than patients who did not receive chemotherapy. This indicates that this patient subset featured various risk factors at initial diagnosis or after surgery, which led physicians to add chemotherapy. After multivariate analysis, chemotherapy showed no prognostic significance.
In subgroup analysis, no subgroups were identified to have benefit from ENT in terms of survival outcomes or regional failure rates. All tumor sites showed no statistically significant differences according to addition of ENT in OS and PFS. All histologic subtypes (squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma) and T stages (T2-3, T4 stage) also mostly showed similar results in OS and PFS. However, this result should be carefully interpreted. It is highly likely that patients with known risk factors of locoregional recurrences would have received ENT (+), which could have resulted in bias in this retrospective study. The finding of worse PFS even with ENT in subgroup of adenoid cystic carcinoma and T2-3 stage might be explained by this bias. Although statistically significant difference of patient and tumor characteristics between the ENT (+) and the ENT (−) group in each subgroup was not found due to small number of patients, it is possible that the effect of ENT on survival outcomes and RF rates was masked by this bias.
On the other hand, despite the inefficacy of ENT on survival outcomes and pattern of failure shown in our study, patients with RF clearly had dismal prognosis compared with patients who did not have RF. The 5-year OS and PFS rates were 41% and 14%, respectively, in the RF (+) group, which were significantly worse than those of the RF (−) group (p < 0.01). A notable point was that the survival outcomes in the RF (+) group were similar to those in the DF (+) group. The worse prognosis of patients with RF than that of patients without RF has been previously reported in several studies. Paulino et al. [17] found a statistically significant difference in OS rates between the RF (+) and RF (−) groups. A similar pattern was also discovered in a retrospective review of maxillary sinus cancer, showing 5-year survival of 37% in patients with neck control, and 0% in patients with neck relapse [11]. Another study also pointed out that the prognosis of RF patients was similar to that of patients with T4N0 disease [10]. According to our study, patients who experienced RF mostly died of organ failure caused by subsequent distant metastasis or combined local disease progression. The dismal prognosis of patients with RF and possible bias in our study emphasizes that efforts should continue to find a specific patient subgroup in this disease that would benefit from ENT, to improve survival in this disease, yet it has not been found in present study.
Our study is a retrospective review, and thus has innate limitations. First, some specific surgical pathology characteristics (e.g., RM, PNI, LVI status) were missing. Among patients who underwent surgery, many previous pathology reports have lacked information on those characteristics. As a result, analysis based on specific pathology characteristics was limited. In addition, PNS cancers other than maxillary sinus (ethmoid sinus, n = 13; sphenoid sinus, n = 2) or pathology other than squamous cell carcinoma (adenocarcinoma, n = 5; adenoid cystic carcinoma, n = 23) might have been insufficient in number to show any statistically significant difference in subgroup analysis. However, although the number of patients was small, subgroup analysis with specific tumor site or pathology, including analysis excluding adenoid cystic carcinoma, did not show different outcomes. Also, the strength of the present study is quite clear in that it is the one of the few studies directly comparing the efficacy of ENT in patients with PNS cancer whose initial clinical diagnosis was N0, with respect to survival and pattern of failure.
In conclusion, our retrospective review indicated that ENT might have no apparent benefit regarding OS, PFS, pattern of failure, as well as RF rates, in patients with cN0 PNS cancer. ENT should not be generalized to all patients with cN0 PNS cancer. This treatment should only be considered carefully in patients with many adverse features after multidisciplinary discussion. Instead, local control should be considered first; our result showing that RM status is a statistically significant prognostic factor for survival supports this idea. However, the dismal prognosis of patients after RF and the limitations of this retrospective study emphasize the need for further research with larger cohorts, to identify the specific patient subset that would benefit from ENT.
Fig. 1.Treatment flow chart of patients with clinically N0 (cN0) paranasal sinus (PNS) cancer, with or without elective neck treatment (ENT). 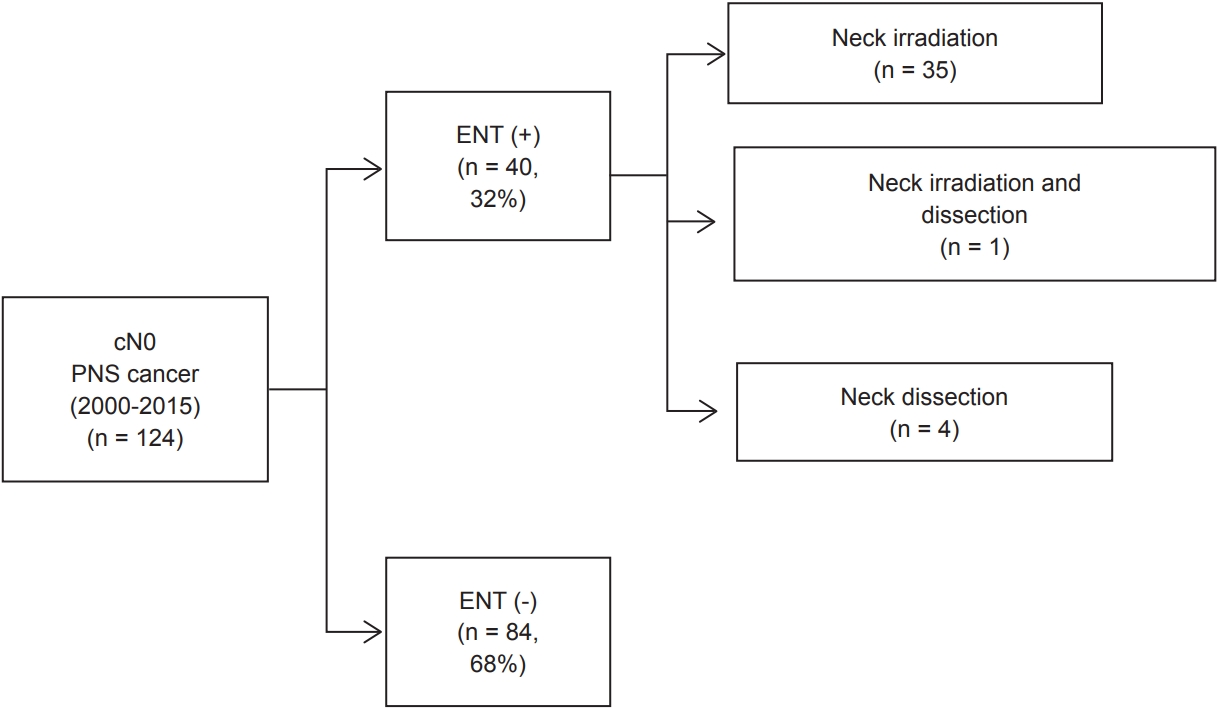
Fig. 2.Overall survival (OS) and progression-free survival (PFS) rates in all patients with clinically N0 paranasal sinus cancer (n = 124). 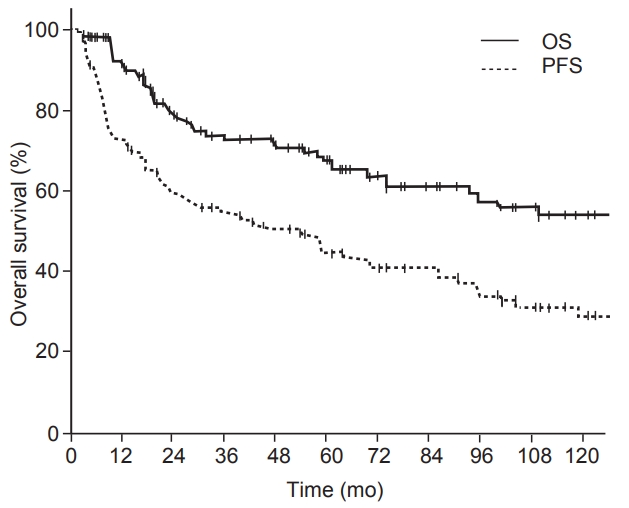
Fig. 3.Comparison of Kaplan-Meier survival curves regarding elective neck treatment (ENT): overall survival (A) and progression-free survival (B). 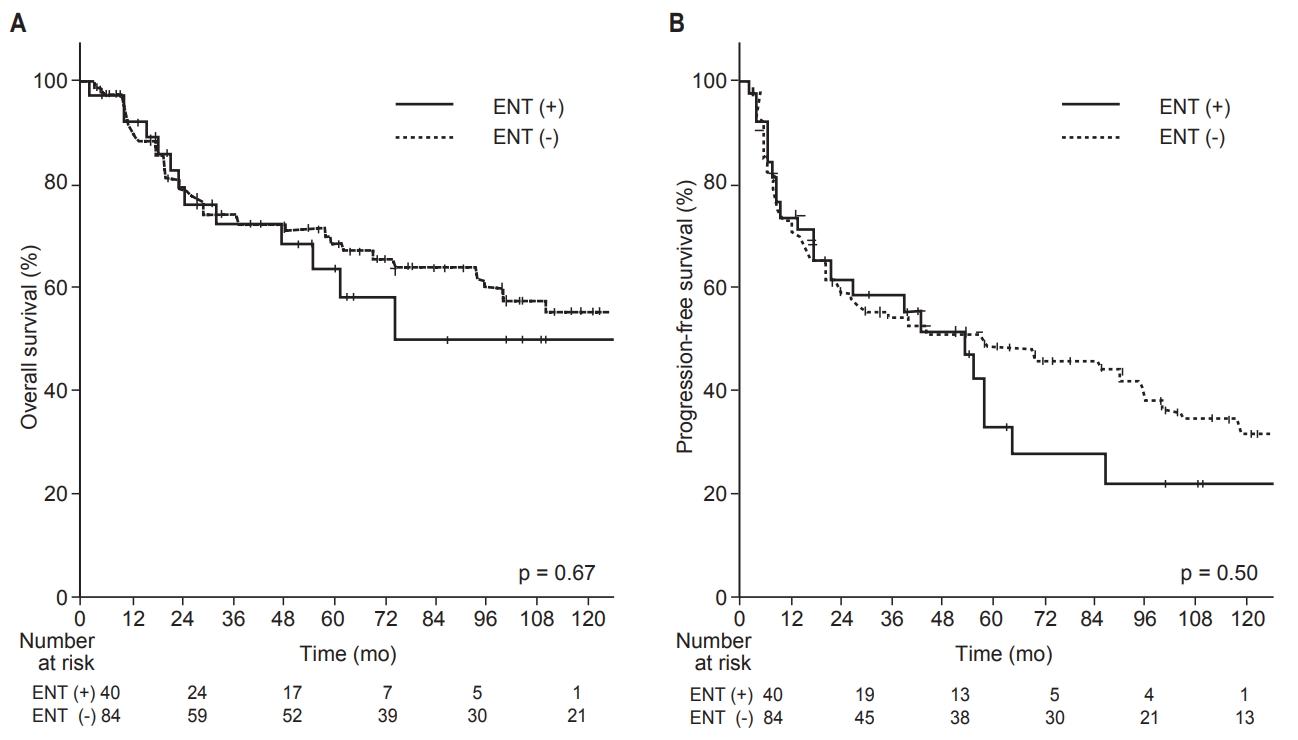
Fig. 4.Regional failure pattern in the ENT (−) group: ipsilateral neck (A) and contralateral neck (B). 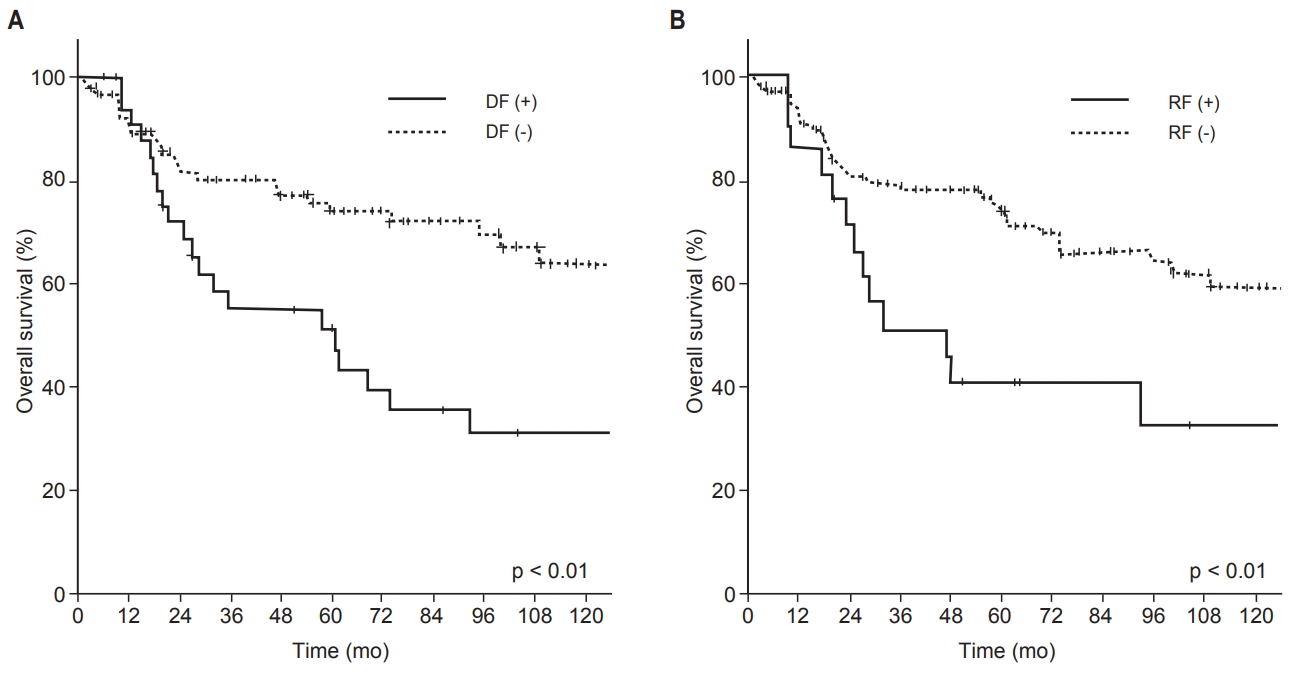
Fig. 5.Comparison of Kaplan-Meier survival curves of overall survival with respect to distant failure (A) and regional failure (B). 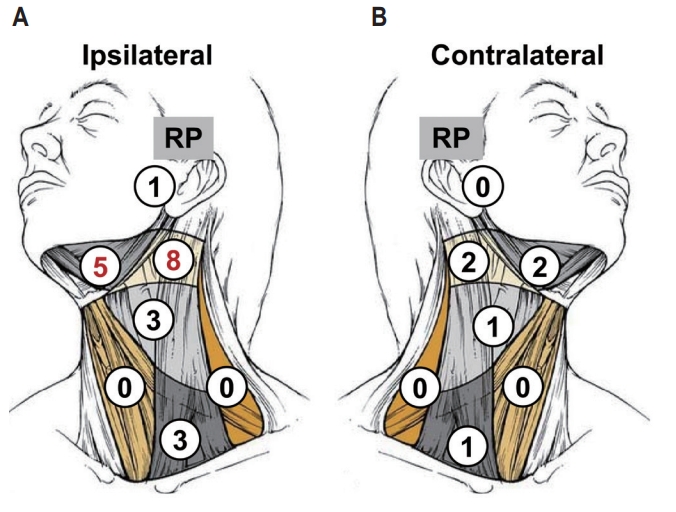
Fig. 6.Comparison of Kaplan-Meier survival curves of subgroup of maxillary sinus: overall survival (A) and progression-free survival (B). ENT, elective neck treatment. 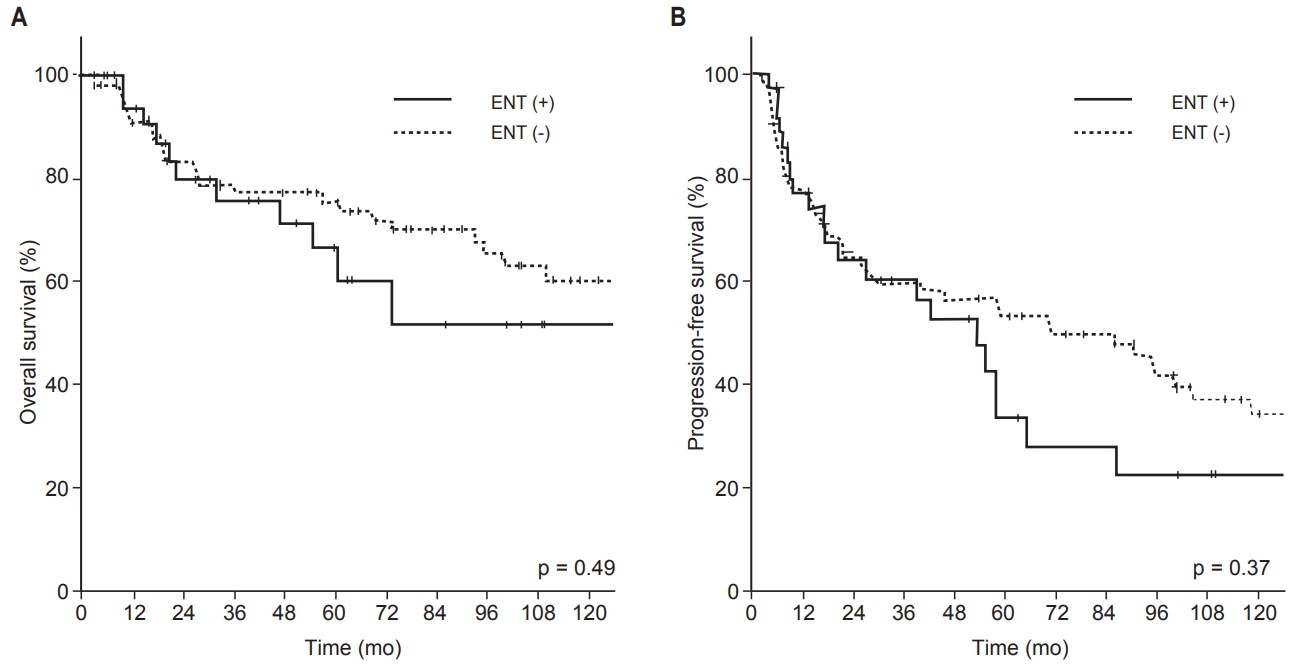
Fig. 7.Comparison of Kaplan-Meier survival curves of subgroup of squamous cell carcinoma: overall survival (A) and progression-free survival (B). ENT, elective neck treatment. 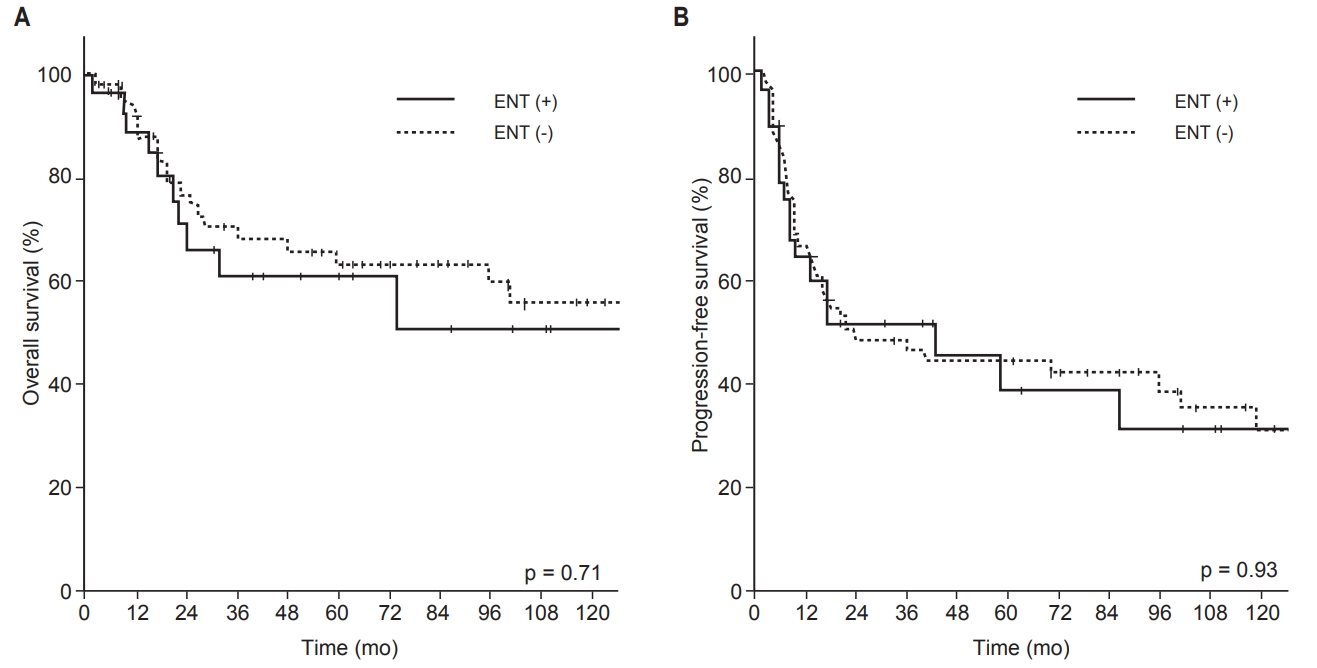
Table 1.Patient and tumor characteristics
Values are presented as median (range) or number (%). ENT, elective neck treatment; Op, operation; CTx, chemotherapy; RT, radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; RM, resection margin; PNI, perineural invasion; LVI, lymphovascular invasion. Table 2.Univariate and multivariate analyses of OS and PFS Table 3.Patterns of failure according to ENT
Table 4.Details of patients with regional failure in the ENT (−) group ENT, elective neck treatment; SCCa, squamous cell carcinoma; ACCa, adenoid cystic carcinoma; CTx, chemotherapy; RT, radiotherapy; RP, retropharyngeal; DDP, cisplatin; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; RM, resection margin; PNI, perineural invasion; LVI, lymphovascular invasion. Table 5.Subgroup analysis of OS and PFS References2. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 2012;34:877–85.
3. Paulino AC, Marks JE, Bricker P, Melian E, Reddy SP, Emami B. Results of treatment of patients with maxillary sinus carcinoma. Cancer 1998;83:457–65.
4. Jansen EP, Keus RB, Hilgers FJ, Haas RL, Tan IB, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys 2000;48:27–35.
5. Kang JH, Cho SH, Kim JP, et al. Treatment outcomes between concurrent chemoradiotherapy and combination of surgery, radiotherapy, and/or chemotherapy in stage III and IV maxillary sinus cancer: multi-institutional retrospective analysis. J Oral Maxillofac Surg 2012;70:1717–23.
6. Cantu G, Bimbi G, Miceli R, et al. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg 2008;134:170–7.
7. Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck 2002;24:821–9.
8. Homma A, Hayashi R, Matsuura K, et al. Lymph node metastasis in t4 maxillary sinus squamous cell carcinoma: incidence and treatment outcome. Ann Surg Oncol 2014;21:1706–10.
9. Pezner RD, Moss WT, Tong D, Blasko JC, Griffin TW. Cervical lymph node metastases in patients with squamous cell carcinoma of the maxillary antrum: the role of elective irradiation of the clinically negative neck. Int J Radiat Oncol Biol Phys 1979;5:1977–80.
10. Kim GE, Chung EJ, Lim JJ, et al. Clinical significance of neck node metastasis in squamous cell carcinoma of the maxillary antrum. Am J Otolaryngol 1999;20:383–90.
11. Le QT, Fu KK, Kaplan MJ, Terris DJ, Fee WE, Goffinet DR. Lymph node metastasis in maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys 2000;46:541–9.
12. Jiang GL, Ang KK, Peters LJ, Wendt CD, Oswald MJ, Goepfert H. Maxillary sinus carcinomas: natural history and results of postoperative radiotherapy. Radiother Oncol 1991;21:193–200.
13. Joosten M, de Bree R, Van Cann EM. Management of the clinically node negative neck in squamous cell carcinoma of the maxilla. Oral Oncol 2017;66:87–92.
14. Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014;110:172–81.
15. Park JH, Nam W, Kim HJ, Cha IH. Is elective neck dissection needed in squamous cell carcinoma of maxilla? J Korean Assoc Oral Maxillofac Surg 2017;43:166–70.
16. Jeon SH, Han DH, Won TB, Keam B, Kim JH, Wu HG. Implication of tumor location for lymph node metastasis in maxillary sinus carcinoma: indications for elective neck treatment. J Oral Maxillofac Surg 2017;75:858–66.
17. Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys 1997;39:283–9.
18. Yang Z, Deng R, Sun G, Huang X, Tang E. Cervical metastases from squamous cell carcinoma of hard palate and maxillary alveolus: a retrospective study of 10 years. Head Neck 2014;36:969–75.
19. Jang NY, Wu HG, Park CI, et al. Definitive radiotherapy with or without chemotherapy for T3-4N0 squamous cell carcinoma of the maxillary sinus and nasal cavity. Jpn J Clin Oncol 2010;40:542–8.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |