 |
 |
AbstractPurposeThe purpose of this study was to determine if somatic mutations are associated with clinical and pathologic outcomes in patients with borderline resectable pancreatic cancer (BRPC) or locally advanced pancreatic cancer (LAPC) who were treated with neoadjuvant chemotherapy and stereotactic body radiotherapy (SBRT).
Materials and MethodsPatients treated with neoadjuvant chemotherapy and SBRT followed by surgical resection from August 2016 to January 2019 and who underwent next generation sequencing of their primary tumor were included in the study. Next-generation sequencing was performed either in-house with a Solid Tumor Panel or with FoundationOne CDx. Univariate (UVA) and multivariable analyses (MVA) were performed to determine associations between somatic mutations and pathologic and clinical outcomes.
ResultsThirty-five patients were included in the study. Chemotherapy consisted of modified FOLFIRINOX, gemcitabine and nab-paclitaxel, or gemcitabine and capecitabine. Patients were treated with SBRT in 33 Gy in 5 fractions. On UVA and MVA, tumors with KRAS G12V mutation demonstrated better pathologic tumor regression grade (TRG) to neoadjuvant therapy when compared to tumors with other KRAS mutations (odds ratio = 0.087; 95% confidence interval [CI], 0.009ŌĆō0.860; p = 0.036). On UVA and MVA, mutations in NOTCH1/2 were associated with worse overall survival (hazard ratio [HR] = 4.15; 95% CI, 1.57ŌĆō10.95; p = 0.004) and progression-free survival (HR = 3.61; 95% CI, 1.41ŌĆō9.28; p = 0.008). On UVA, only mutations in NOTCH1/2 were associated with inferior distant metastasis-free survival (HR = 3.38; 95% CI, 1.25ŌĆō9.16; p = 0.017).
IntroductionPancreatic cancer is the third most common cause of cancer related deaths in the United States and accounts for roughly 47,000 deaths each year [1]. At time of diagnosis, 10% are resectable, 40% are borderline resectable pancreatic cancer (BRPC) or locally advanced pancreatic cancer (LAPC), and 50% are metastatic. Treatment of pancreatic cancer depends on disease extent and includes a combination of chemotherapy, radiation therapy, and surgical resection [2]. Unfortunately, even with modern treatment, prognosis of BRPC and LAPC is poor with 5-year overall survival (OS) of less than 15% [1,3].
The management of BRPC and LAPC is complex with the majority of patients treated with upfront chemotherapy followed by surgery if technically feasible. Radiation therapy can be administered with the goal of margin sterilization and local recurrence risk reduction in the neoadjuvant setting and local progression-free survival benefit in the unresectable setting, but its role remains controversial [4-9]. Indeed, several large randomized control studies have investigated the use radiation therapy in BRPC and LAPC with mixed results, which may in part be related to variable radiosensitivity due to molecularly heterogeneous disease biology [5-9]. Certainly, tools that could better predict therapeutic response in this group of patients would be helpful.
In recent years, there has been an increased role of molecular testing in pancreatic cancer. The National Comprehensive Cancer Network (NCCN) guidelines now recommend somatic mutation testing for all metastatic and locally advanced disease [2]. It is thought that the poor prognosis of pancreatic cancer may in part be due to tumor heterogeneity at the molecular level, with a wide variety of mutations present [10-14]. Studies have shown that mutational status is associated with clinical outcomes and that targeted therapy may improve prognosis [15-20]. However, many of these reports included heterogeneous patient populations who were treated with various local and systemic therapies. Furthermore, these studies do not report on pathologic tumor response. As such, we herein explore the impact of somatic mutations on clinical and pathologic outcomes in a cohort of BRPC and LAPC patients treated with stereotactic body radiotherapy (SBRT) after upfront chemotherapy, reporting on both pathologic tumor response to neoadjuvant chemotherapy and radiation as well as on survival outcomes.
Materials and Methods1. Study designThis is a single institution retrospective review of patients with localized pancreatic cancer who were treated from August 2016 to January 2019 with chemotherapy, SBRT, and surgical resection and who underwent next-generation sequencing (NGS) of the primary tumor specimen. This study was approved by the Institutional Review Board of Johns Hopkins University School of Medicine (No. IRB00270193). The informed consent was waived given that this was a retrospective study and no human experimentation/interaction was performed. Patients were included in the study if they met the following criteria: (1) biopsy proven diagnosis of pancreatic cancer; (2) BRPC and LAPC as defined by NCCN guidelines [2]; (3) treatment with neoadjuvant chemotherapy and SBRT followed by surgical resection; (4) adequate follow-up defined as >3 clinical encounters following treatment; and (5) NGS of tumor specimen. Routine follow-up consisted of clinic visits at approximately 3-month intervals and pancreatic protocol imaging performed at 3ŌĆō6 months intervals. As part of follow-up, patients had cancer antigen 19-9 (CA19-9) levels drawn. The frequency of imaging and blood work were at the discretion of the clinical team.
2. Treatment detailsPatients were treated with upfront chemotherapy with modified FOLFIRINOX (mFFX), gemcitabine and nab-paclitaxel (GnP), or gemcitabine and capecitabine. During chemotherapy, patients had pancreatic protocol computed tomography (CT) scans every 3 months to assess treatment response. After completion of chemotherapy, patients were recommended for SBRT. Historically, at our institution, all patients with BRPC/LAPC who have responding or stable disease after upfront systemic therapy have been offered SBRT to 33 Gy in 5 fractions. Patients were subsequently restaged after SBRT, and a decision was made regarding surgical exploration. In general, all technically BRPC patients were explored if no medical contraindications and no evidence of progression after SBRT [21]. This has similarly been true for the LAPC patients, with the exception of a small minority of LAPC patients for whom local extent of disease was too extensive to offer a reasonable pathway for complete surgical resection [22]. Of note, presence of duodenal invasion on endoscopy has been the primary contraindication to SBRT, for which conventional chemoradiation has been recommended instead. More recently, we have also considered dose-escalated IMRT for the minority of LAPC patients that fit into the category above of too locally extensive disease to allow a reasonable pathway for complete resection [23], but these patients were excluded from this analysis given potential impact on clinical outcomes.
Prior to simulation and SBRT, all patients underwent ultrasound-guided endoscopic gold fiducial placement for the purpose of daily image guidance. At time of simulation, patients were positioned supine with arms above head in a Vac-lok (CIVCO Medical Solutions, Coralville, IA, USA) for immobilization. Thin-sliced CT scans with intravenous contrast were obtained for radiation treatment planning. Motion management with active breathing control (ABC; Elekta, Stockholm, Sweden) was performed in the majority of patients. Patients who could not tolerate breath-hold were treated under free-breathing conditions, with an internal target volume (ITV) generated from the peak inspiratory and expiratory phases from a four-dimensional CT scan. The clinical target volume (CTV) and organs-at-risk were contoured using Pinnacle treatment planning system (Philips Radiation Oncology Systems, Fitchburg, WI, USA). The CTV included gross disease seen on imaging and areas at risk for microscopic disease, namely the full circumference of involved vasculature. The planning target volume was generated by adding a 2-mm isotropic expansion to the CTV for breath-hold cases and to the ITV for free-breathing cases. Daily image guidance with pre-treatment and intrafractional cone-beam CT scans was performed to ensure proper setup. Patients were aligned to spine and then shifted to align to fiducials. All patients were treated on an Elekta Synergy-S unit with HexaPOD evo RT system.
Approximately 4 weeks after completion of SBRT, patients were restaged with imaging, with surgical resection shortly thereafter. Adjuvant or maintenance chemotherapy was initiated at the discretion of the treating medical oncologist.
3. Molecular testingGenomic information was acquired from formalin-fixed paraffin-embedded tissue from surgical specimens. NGS was performed either in-house using a Clinical Laboratory Improvement Amendments certified Johns Hopkins Molecular Lab with a Solid Tumor Panel (STP) [24] or with FoundationOne CDx. The in-house Solid Tumor Panel and FoundationOne CDx included testing for mutations in genes of interest such as KRAS, NOTCH1, NOTCH2, CDKN2A, BRCA1, BRCA2, SMAD4, ATM, as well as in over 300 other genes [25,26]. Genes were considered mutated if they had substitutions, insertions-deletions, or copy-number alterations. Genes that did not harbor these alterations were considered wild-type. Tumor content within extracted samples was >10% for STP and >20% for FoundationOne CDx. Mean sequencing depth was 700├Ś for STP and 894├Ś for FoundationOne CDx.
4. Classification of pathologic responseAt our institution, tumor response to neoadjuvant therapy was graded per the American Joint Committee on Cancer/College of American Pathologist tumor regression grade (TRG) system [27]. No residual tumor or complete response is considered TRG 0, while poor response/no response is considered TRG 3. Marked response with minimal residual cancer with single cells is considered TRG 1, and moderate response with residual cancer outgrown by fibrosis is considered TRG 2.
5. Clinical outcomesClinical outcomes included OS, local progression-free survival (LPFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS). OS was defined as time from surgery to death. LPFS and DMFS were defined as time from surgery to locoregional progression or distant progression, respectively. PFS was defined as time from surgery to death or any radiographic evidence of progression.
6. StatisticsBaseline demographic, tumor, and treatment characteristics were recorded including age, sex, performance status, tumor size, tumor grade, chemotherapy regimen, SBRT dose/fractionation, resection status, and mutational status. Univariate Cox analysis was performed identify variables associated with clinical outcomes from time of surgery. Variables with p < 0.2 on univariate Cox analysis were entered into multivariable Cox analysis and subsequently removed if p-value rose to above 0.2. Kaplan-Meier analysis was performed for time to event outcomes, and log-rank test was used assess significance between groups. Univariate logistic regression was performed to determine associations between variables and TRG. Variables with p < 0.2 on univariate nominal logistic regression were entered into multinomial logistic regression and were subsequently removed if p-value rose to above 0.2. All statistical analyses were performed with JMP version 14.0 (SAS Institute, Cary, NC, USA).
Results1. Patient and disease characteristicsFrom August 2016 to January 2019, 35 patients were treated with neoadjuvant chemotherapy and SBRT followed by surgical resection and underwent NGS of their tumor specimen. Patient and disease characteristics are described in Table 1. Patients underwent NGS of their tumor specimen either in-house with a STP (29/35, 83%) or with FoundationOne CDx (6/35, 17%). The median age at diagnosis was 67 years (range, 46 to 80 years). Borderline resectable disease was seen in 20 patients (57%) and LAPC in 15 patients (43%). Location of the tumor was in the pancreatic head in 15 patients (44%). Median tumor size was 3.6 cm (range, 1.4 to 6.7 cm). Median baseline CA19-9 was 148.6 U/mL (range, 16 to 5,545 U/mL).
2. Treatment characteristicsUpfront chemotherapy regimens consisted of mFFX (23/35, 66%), GnP (5/35 14%), mFFX followed by GnP (5/35 14%), mFFX followed by gemcitabine alone (1/35, 3%), and gemcitabine and capecitabine (1/35, 3%). Median duration of upfront chemotherapy was 4 months (range, 2 to 12 months). Following chemotherapy, all patients were treated with SBRT to 33 Gy in 5 fractions. Median time from SBRT to surgical resection was 6.9 weeks (range, 3.1 to 25.3 weeks). Surgical margins were negative in 32 patients (91%). Pathologically involved lymph nodes were found in 17/35 patients (49%). Whipple procedure was performed in 20 patients (57%), distal pancreatectomy in 14 patients (40%), and total pancreatectomy in one patient (3%). Adjuvant or maintenance chemotherapy was initiated in 26 patients (74%) for a median time of 2 months (range, 1 to 4 months).
3. Frequency of mutations
Fig. 1 displays the frequencies of common mutations and KRAS mutation subtypes. Mutations in KRAS were the most common, present in 33 of 35 patients (94%). KRAS mutations were most frequent at codon 12 (37/41, 90%), with the following subtype frequencies: G12V (14/33, 42%), G12D (13/33, 39%), G12R (3/33, 9%), Q61H (2/33, 6%), and unknown (1/33, 3%). The next most common mutations were in TP53 (21/35, 60%), NOTCH1/2 (8/35, 23%), and CDKN2A (6/35, 17%). Less common mutations included SMAD4 (2/35, 6%), BRCA 1/2 (4/35, 11%), and ATM (2/35, 6%).
4. Clinical outcomesThe median follow-up time from SBRT was 20.4 months (range, 3.5 to 45.4 months). At time of last follow-up, nine of 35 patients (26%) were alive. Median OS from surgery was 24.5 months, with 1-, 2-, and 3-year OS rates of 76.6%, 52.7%, and 10.1%, respectively. The median LPFS, DMFS, and PFS from surgery were 20.2 (range, 2.8 to 44.0 months), 12.2 months (range, 0.5 to 38.1 months), 11.3 months (range, 0.5 to 38.1 months), respectively. The majority of patients (26/35, 74%) had distant disease at time of last follow-up. Local failure was observed in 19 patients (54%), including isolated local failure in two patients (6%). Of the 17 patients who developed local and distant failure, pattern of first failure included distant in 5/17 patients (29.4%), local in 5/17 patients (29.4%), and synchronous local and distant in 7/17 patients (41.2%). Radiographic response following neoadjuvant chemotherapy and SBRT was determined by CT imaging just prior to surgery. Eleven patients (31%) had partial response, while 24 patients (69%) had stable disease. There were no cases of radiographic disease progression.
On univariate (UVA) and multivariable (MVA) analyses, only mutations in NOTCH1/2, were associated with OS (hazard ratio [HR] = 4.15; 95% confidence interval [CI], 1.57ŌĆō10.95; p = 0.004) (Table 2). Patients with NOTCH1/2 mutations had a median OS of 13.9 months versus 27.6 months in NOTCH1/2 wild-type patients (log-rank, p = 0.003) (Fig. 2A). On UVA, only mutations in NOTCH1/2 were associated with DMFS (HR = 3.38; 95% CI, 1.25ŌĆō9.16; p = 0.017) (Table 3). No other variables were associated with DMFS. Patients with NOTCH1/2 mutations had a median DMFS of 7.1 months versus 15.9 months in NOTCH1/2 wild-type patients (log-rank, p = 0.011) (Fig. 2B). Similarly, on UVA and MVA, only mutations in NOTCH1/2 were associated with PFS (HR = 3.61; 95% CI, 1.41ŌĆō9.28; p = 0.008) (Table 4). Patients with NOTCH1/2 2 mutations had a median PFS of 7.1 months versus 12.6 months in NOTCH1/2 wild-type patients (log-rank, p = 0.003) (Fig. 2C). No variables were associated with LPFS (Supplementary Table S1).
Tumor regression following chemotherapy and SBRT was evaluated with respect to various variables, including mutational status (Table 5). One patient did not have TRG information available for review. Tumor regression grade 0 was seen in 1/34 patients (3%), TRG 1 in 7/34 patients (21%), TRG 2 in 20/34 patients (59%), and TRG 3 in 6/34 patients (18%). On univariate and multinomial logistic regression, only KRAS G12V mutational status was associated with TRG. Tumors with KRAS G12V mutation were more likely to demonstrate a marked to complete response (TRG 0ŌĆō1) to chemotherapy and SBRT when compared to all other KRAS mutations (odds ratio = 0.087; 95% CI, 0.009ŌĆō0.860; p = 0.036) (Table 5). Among patients with the KRAS G12V mutation, 6/14 patient (43%) achieved a marked to complete tumor response (TRG 0ŌĆō1) compared to just 2/19 patients (11%) with KRAS non-G12V mutations (Pearson chi-square, p = 0.031) (Table 6). Only one patient in the entire cohort demonstrated a complete response (TRG 0) to chemotherapy and SBRT, and this patientŌĆÖs tumor harbored the KRAS G12V mutation. Of the 14 patients with KRAS G12V mutations, TRG 0 was achieved in one patient (7%), TRG 1 in five patients (36%), and TRG 2 in eight patients (57%). No tumors with KRAS G12V mutation demonstrated no/poor response (TRG 3). Table 6 shows TRG for KRAS G12V and non-G12V subtypes.
Discussion and ConclusionTo our knowledge, this is the first study to report on the impact of somatic mutations in a cohort of BRPC and LAPC patients treated with SBRT after upfront chemotherapy. We show that mutational status is an important predictor of clinical and pathologic outcomes. Specifically, the KRAS G12V mutation was associated with more favorable TRG following chemotherapy and SBRT when compared to other KRAS mutations. Additionally, mutations in NOTCH1/2 were associated with inferior OS, DMFS, and PFS.
Localized pancreatic cancer has a high rate of distant failure after surgical resection, highlighting the need for more effective systemic therapy [28]. However, local failure is not insignificant with approximately 30% of patients developing isolated local failure without distant disease after resection [28,29]. In fact, up to 30% of LAPC patients die from locally destructive disease [30]. Therefore, more effective local therapy such as radiation is needed. However, the use of radiation therapy in BRPC and LAPC remains contentious [5-9]. A phase II/III study from Korea demonstrated that neoadjuvant chemoradiation was associated with improved R0 resection rate and 2-year OS when compared to upfront surgery in BRPC [5]. The PREOPANC trial showed similar findings, with higher rates of R0 resection with neoadjuvant chemoradiation compared to upfront surgery [6]. However, the more recent ALLIANCE 021501 trial demonstrated contradictory findings, with lower R0 rates and worse 18-month OS in BRPC treated with neoadjuvant chemoradiation versus neoadjuvant chemotherapy alone [7]. Mixed findings are also seen in LAPC, with the LAP07 trial showing no OS benefit but an improvement in local control with consolidative chemoradiation compared to consolidative chemotherapy alone [8]. These varied findings may in part be due to the heterogeneous molecular biology of pancreatic cancer [10-14].
Somatic mutations in pancreatic cancer are more prevalent and diverse than previously realized [10-14]. Over 95% of pancreatic cancer have genetic alterations including translocations, frameshifts, deletions, insertions, and substitutions [11]. In fact, the NCCN guidelines now recommend somatic gene profiling for all patients with LAPC or metastatic disease who are candidates for systemic therapy [2]. Of most interest are actionable mutations including BRAF, BRCA 1/2, HER2, KRAS, and PALB2, which can dictate response to systemic therapy. In other cancer types, specific mutations can also predict radiosensitivity [31]. Certainly, it would be of value to identify mutations associated with radiosensitivity in BRPC and LAPC.
Although it is impossible to differentiate the relative contribution of chemotherapy versus SBRT to pathologic response, TRG may nonetheless serve as a marker of sensitivity to both chemotherapy and radiation. Our data suggest that BRPC and LAPC with the KRAS G12V mutation are more likely to achieve a marked to complete response (TRG 0ŌĆō1) to chemotherapy and SBRT when compared to tumors with other KRAS mutations. This is consistent with data from early stage non-small-cell lung carcinoma (NSCLC) treated with surgery alone and from advanced stage NSCLC treated with platinum-based chemotherapy, with G12V tumors performing better in both studies [32,33]. However, our finding is contradictory to a report on rectal cancer patients treated with preoperative chemoradiation, with G12V associated with lower rates of tumor regression, although not statistically significant [34]. This discrepancy may be attributed to differences in radiation dose fractionation, systemic therapy, and tumor biology. If the variation in TRG response based on KRAS mutational subtype is driven by variation in radiosensitivity, our findings may have implications on selection of radiation dose based on KRAS mutation subtype. Recent data has highlighted the importance of radiation dose in LAPC, with biologically effective doses >70 Gy leading to improved OS [35]. It may follow therefore that patients who are less likely to achieve adequate pathologic response, such as those with KRAS non-G12V mutations in our study, may be ideal candidates for radiation dose escalation strategies to improve outcomes. Conversely, patients who are more likely to achieve a good pathologic response may be able to be spared from the potential side effects of increased radiation dose. Certainly, further studies are warranted in determining the impact of KRAS mutational subtype on local response to SBRT and chemotherapy in localized pancreatic cancer.
Notch proteins are a group of highly conserved transmembrane receptors responsible cellular proliferation, differentiation, and apoptosis [36-38]. Aberrant Notch signaling leads to a wide range of disorders including cancer [39]. Rossi et al. [40] showed that NOTCH1 mutations are an independent predictor of worse OS in chronic lymphocytic leukemia (median OS, 3.5 vs. 13.9 months; p < 0.05). Similar results were reported by Zhu et al. [41] in adult T-cell acute lymphoblastic patients with NOTCH1 mutations (3-year OS, 31.8% vs. 71.7%; p < 0.05). Notch expression has been implicated in pancreatic adenocarcinoma, with increased expression leading to poor survival [42]. This is consistent with studies showing that Notch signaling is required for the progression of pancreatic intraepithelial neoplasia to pancreatic adenocarcinoma and that alterations in this pathway can lead to malignancy [40,43]. Notch signaling has also been associated with radio-resistance and radiation-induced epithelial-mesenchymal transition, a process crucial in tumorigenesis [44-46]. Here, we demonstrate that BRPC and LAPC with NOTCH1/2 mutations have worse OS and DMFS when compared to NOTCH1/2 wild-type tumors. These findings suggest that patients with NOTCH1/2 mutations may derive greater benefit from optimization of systemic therapy, as opposed to local therapy such as radiation and surgery, given their high risk of developing distant progression. In fact, several phase I/II studies have investigated the use of ╬│-secretase inhibitors, novel molecules that inhibit Notch signaling, in both metastatic and locally advanced pancreatic cancer [47,48]. Additional studies are needed to further define the mechanism of NOTCH1/2 signaling and how such information can be incorporated into clinical decision-making
Lastly, although not the focus of our study, it is interesting note that 54% of patients developed locoregional failure (LF). Even more interesting was that this rate of LF occurred despite with a 91% R0 resection. Our findings are consistent with a phase II trial by Kharofa et al. [50], who investigated the use of neoadjuvant chemotherapy and SBRT in resectable and BRPC. Locoregional failure occurred in 50% of patients despite R0 resection in 92%. In their study, treatment volumes included gross disease and adjacent vasculature but not elective nodal regions. All LFs occurred out-of-field, which is relevant given recent data highlighting the importance of elective nodal irradiation [51]. Similarly, we did not perform elective nodal irradiation in our patients, which likely contributed to higher LF rates. Indeed, target volume delineation remains highly variable for patients with pancreatic cancer, and more data regarding patterns of local failure are needed to determine optimal field design [51]. Certainly, optimal dose is equally uncertain, given recent data supporting the value of dose-escalation [23].
There are several limitations of this study including its retrospective design and small sample size of 35 patients. This certainly impacts the strength of the findings and precluded us from identifying other potential associations between mutational status and outcomes. For example, although NOTCH1/2 status was associated with clinical outcomes, it was not predictive of pathologic response. Furthermore, prior studies show that KRAS mutations are associated with poor clinical outcomes, but we were unable to detect this relationship as only two patients were KRAS wild-type in our cohort [52,53]. Although we demonstrate that the KRAS G12V mutation predicted for better TRG, there was no association with local control, which may be a better marker of radiation efficacy. We are also unable to comment on whether pathologic response was due to the effect of SBRT, chemotherapy or a combination of both. Additionally, the detection of specific mutations may have been limited by heterogeneity of tumor content in the extracted samples. Finally, we selected for BRPC/LAPC patients who were able to undergo resection, which certainly may present selection biases. Ultimately, prospective validation within and across stage groupings should be pursued. Therefore, these findings should be interpreted with caution and should be validated in a larger cohort of patients. Nonetheless, the results provide valuable information regarding the effect of somatic mutations in pancreatic cancer and is consistent with findings from other studies.
This is the first series to report on the impact of somatic mutations in cohort of BRPC and LAPC treated with SBRT after upfront chemotherapy. We show that the KRAS G12V mutation is associated with better TRG after preoperative chemotherapy and SBRT and that mutations in NOTCH1/2 are independently associated with inferior OS, DMFS, and PFS. These findings suggest that the mutational landscape of pancreatic cancer is important in stratifying patients, and as a result, may help clinicians in choosing targeted therapy.
Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2021.00815.
Table┬ĀS1.Univariate and multivariable analyses of local progression-free survival Fig.┬Ā1.(A) Box chart showing frequency of common mutations and (B) pie chart showing frequency of KRAS mutation subtypes. 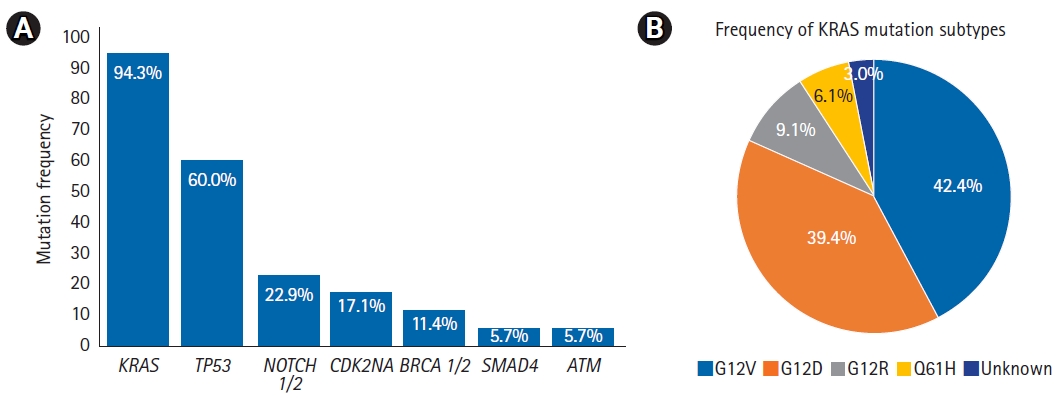
Fig.┬Ā2.Kaplan-Meier curves of (A) overall survival, (B) distant metastasis-free survival, and (C) progression-free survival based on NOTCH1/2 mutation status. 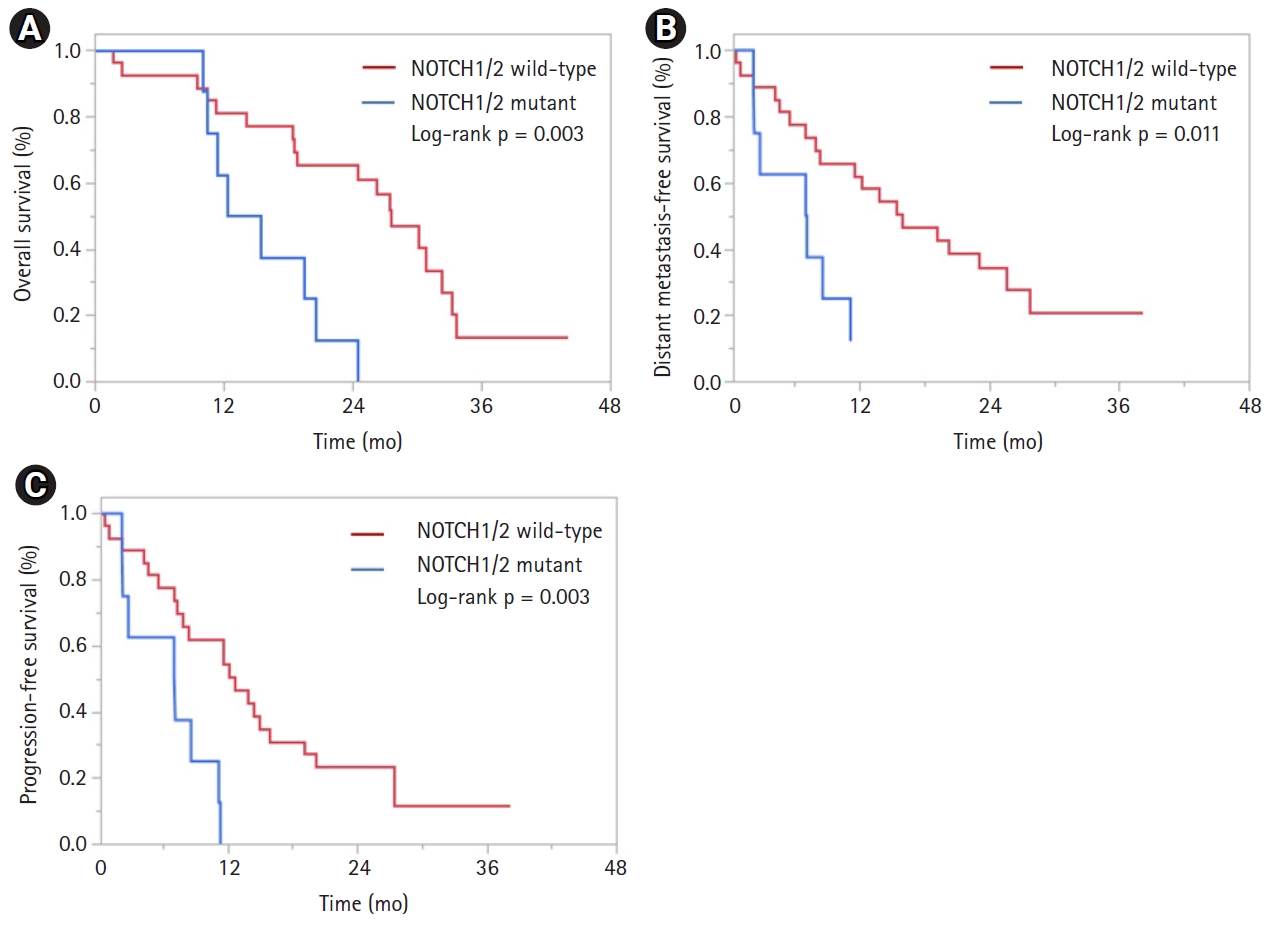
Table┬Ā1.Patient, treatment, and tumor characteristics (n = 35) Table┬Ā2.Univariate and multivariable analyses of overall survival
Table┬Ā3.Univariate and multivariable analyses of distant metastasis-free survival
Table┬Ā4.Univariate and multivariable analyses of progression-free survival
Table┬Ā5.Univariate and multivariable analyses of tumor regression grade 0-1 vs. 2-3
Table┬Ā6.Tumor regression grade based on KRAS G12V mutational status
References2. National Comprehensive Cancer Network. NCCN Guidelines: pancreatic adenocarcinoma version 2.2021 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2021 [cited 2021 Nov 15]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455.
3. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019;10:10ŌĆō27.
4. Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979ŌĆō85.
5. Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 2018;268:215ŌĆō22.
6. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020;38:1763ŌĆō73.
7. Katz MH, Shi Q, Meyers JP, et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol 2021;39(3_Suppl):377.
8. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844ŌĆō53.
9. Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592ŌĆō9.
10. Cros J, Raffenne J, Couvelard A, Pote N. Tumor heterogeneity in pancreatic adenocarcinoma. Pathobiology 2018;85:64ŌĆō71.
11. Cicenas J, Kvederaviciute K, Meskinyte I, Meskinyte-Kausiliene E, Skeberdyte A, Cicenas J. KRAS, TP53, CDKN2A, SMAD4, BRCA1, and BRCA2 mutations in pancreatic cancer. Cancers (Basel) 2017;9:42.
12. Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res 1988;16:7773ŌĆō82.
13. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47ŌĆō52.
14. Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500ŌĆō3.
15. Windon AL, Loaiza-Bonilla A, Jensen CE, Randall M, Morrissette J, Shroff SG. A KRAS wild type mutational status confers a survival advantage in pancreatic ductal adenocarcinoma. J Gastrointest Oncol 2018;9:1ŌĆō10.
16. Kim ST, Lim DH, Jang KT, et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther 2011;10:1993ŌĆō9.
17. Rachakonda PS, Bauer AS, Xie H, et al. Somatic mutations in exocrine pancreatic tumors: association with patient survival. PLoS One 2013;8:e60870.
18. Yu S, Agarwal P, Mamtani R, et al. Retrospective survival analysis of patients with resected pancreatic ductal adenocarcinoma and a germline BRCA or PALB2 mutation. JCO Precis Oncol 2019;3:00271.
19. Bournet B, Muscari F, Buscail C, et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma. Clin Transl Gastroenterol 2016;7:e157.
20. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 2020;21:508ŌĆō18.
21. Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg 2019;270:340ŌĆō7.
22. Javed AA, Wright MJ, Siddique A, et al. Outcome of patients with borderline resectable pancreatic cancer in the contemporary era of neoadjuvant chemotherapy. J Gastrointest Surg 2019;23:112ŌĆō21.
23. Reyngold M, O'Reilly EM, Varghese AM, et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol 2021;7:735ŌĆō8.
24. Ding D, Javed AA, Cunningham D, et al. Challenges of the current precision medicine approach for pancreatic cancer: a single institution experience between 2013 and 2017. Cancer Lett 2021;497:221ŌĆō8.
25. FoundationOne CDx Technical Information [Internet]. Cambridge, MA: Foundation Medicine Inc.; 2021 [cited 2021 Nov 15]. Available from: https://info.foundationmedicine.com/hubfs/FMI%20Labels/FoundationOne_CDx_Label_Technical_Info.pdf.
26. Solid Tumor Panel Gene List v6.0 [Internet]. Baltimore, MD: Johns Hopkins Molecular Diagnostics Laboratory; 2021 [cited 2021 Nov 15]. Available from: https://pathology.jhu.edu/jhml-services/assets/test-directory/SolidTumorPanel-II_GeneList_v6.0.pdf.
27. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010.
28. Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600ŌĆō4.
29. Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 2006;10:511ŌĆō8.
30. Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806ŌĆō13.
31. Yard BD, Adams DJ, Chie EK, et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun 2016;7:11428.
32. Izar B, Zhou H, Heist RS, et al. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol 2014;9:1363ŌĆō9.
33. Cserepes M, Ostoros G, Lohinai Z, et al. Subtype-specific KRAS mutations in advanced lung adenocarcinoma: a retrospective study of patients treated with platinum-based chemotherapy. Eur J Cancer 2014;50:1819ŌĆō28.
34. Gaedcke J, Grade M, Jung K, et al. KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother Oncol 2010;94:76ŌĆō81.
35. Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755ŌĆō65.
36. Sato C, Zhao G, Ilagan MX. An overview of notch signaling in adult tissue renewal and maintenance. Curr Alzheimer Res 2012;9:227ŌĆō40.
37. Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol 2017;12:245ŌĆō75.
38. Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett 2015;369:20ŌĆō7.
39. Avila JL, Kissil JL. Notch signaling in pancreatic cancer: oncogene or tumor suppressor? Trends Mol Med 2013;19:320ŌĆō7.
40. Rossi D, Rasi S, Fabbri G, et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood 2012;119:521ŌĆō9.
41. Zhu YM, Zhao WL, Fu JF, et al. NOTCH1 mutations in T-cell acute lymphoblastic leukemia: prognostic significance and implication in multifactorial leukemogenesis. Clin Cancer Res 2006;12:3043ŌĆō9.
42. Ye J, Wen J, Ning Y, Li Y. Higher notch expression implies poor survival in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Pancreatology 2018;18:954ŌĆō61.
43. Mazur PK, Einwachter H, Lee M, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 2010;107:13438ŌĆō43.
44. Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010;28:17ŌĆō28.
45. Koerdel K, Spitzner M, Meyer T, et al. NOTCH activation via gp130/STAT3 signaling confers resistance to chemoradiotherapy. Cancers (Basel) 2021;13:455.
46. Kim RK, Kaushik N, Suh Y, et al. Radiation driven epithelial-mesenchymal transition is mediated by Notch signaling in breast cancer. Oncotarget 2016;7:53430ŌĆō42.
47. Richter S, Bedard PL, Chen EX, et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest New Drugs 2014;32:243ŌĆō9.
48. De Jesus-Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739ŌĆō45.
49. Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516ŌĆō22.
50. Kharofa J, Mierzwa M, Olowokure O, et al. Pattern of marginal local failure in a phase II trial of neoadjuvant chemotherapy and stereotactic body radiation therapy for resectable and borderline resectable pancreas cancer. Am J Clin Oncol 2019;42:247ŌĆō52.
51. Miller JA, Toesca D, Baclay J, et al. Pancreatic stereotactic body radiation therapy with or without hypofractionated elective nodal irradiation. Int J Radiat Oncol Biol Phys 2021;Aug 1 [Epub]. https://doi.org/10.1016/j.ijrobp.2021.07.1698.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |