Definitive concurrent chemoradiotherapy in locally advanced pancreatic cancer
Article information
Abstract
Purpose
Survival outcome of locally advanced pancreatic cancer has been poor and little is known about prognostic factors of the disease, especially in locally advanced cases treated with concurrent chemoradiation. This study was to analyze overall survival and prognostic factors of patients treated with concurrent chemoradiotherapy (CCRT) in locally advanced pancreatic cancer.
Materials and Methods
Medical records of 34 patients diagnosed with unresectable pancreatic cancer and treated with definitive CCRT, from December 2003 to December 2012, were reviewed. Median prescribed radiation dose was 50.4 Gy (range, 41.4 to 55.8 Gy), once daily, five times per week, 1.8 to 3 Gy per fraction.
Results
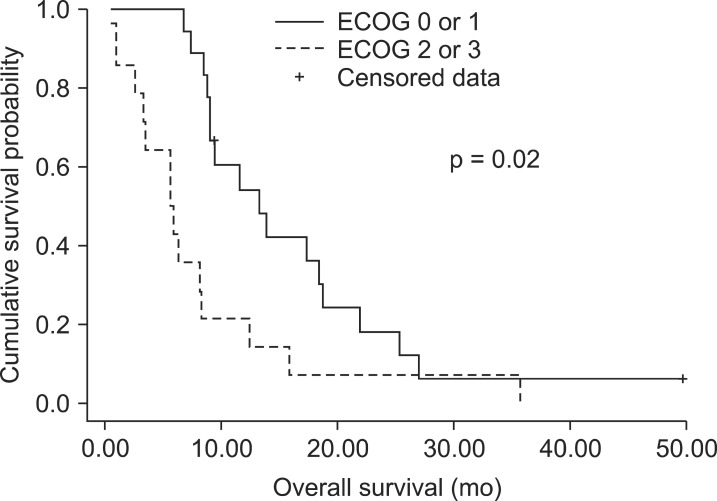
With a mean follow-up of 10 months (range, 0 to 49 months), median overall survival was 9 months. The 1- and 2-year survival rates were 40% and 10%, respectively. Median and mean time to progression were 5 and 7 months, respectively. Prognostic parameters related to overall survival were post-CCRT CA19-9 (p = 0.02), the Eastern Cooperative Oncology Group (ECOG) status (p < 0.01), and radiation dose (p = 0.04) according to univariate analysis. In multivariate analysis, post-CCRT CA19-9 value below 180 U/mL and ECOG status 0 or 1 were statistically significant independent prognostic factors associated with improved overall survival (p < 0.01 and p = 0.02, respectively).
Conclusion
Overall treatment results in locally advanced pancreatic cancer are relatively poor and few improvements have been accomplished in the past decades. Post-treatment CA19-9 below 180 U/mL and ECOG performance status 0 and 1 were significantly associated with an improved overall survival.
Introduction
According to the National Cancer Information Center of Korea, pancreatic cancer accounts for 2.3% of all cancer incidences and it is the 9th most common cancer in both men and women and 5th most common cause related to cancer death. Without clear early symptoms and with fast spread of the disease, more than 80% of pancreatic cancer is locally advanced at presentation [1]. Prognosis of locally advanced and unresectable pancreatic cancer is poor with a median survival of 6 to 10 months [2]. Treatment options for these cancers are chemotherapy, radiotherapy, chemoradiation, and palliative surgery. The Gastrointestinal Tumor Study Group reported prolonged survival using concurrent chemoradiation, with a median survival of 10 months, compared to radiotherapy alone [3]. Along with other trials, [4,5] the study established chemoradiation as standard for patients with locally advanced pancreatic cancer.
Many studies in the past several decades have reported on different strategies concerning treatments of locally advanced pancreatic cancer. Most commonly used chemotherapeutic agents are 5-fluorouracil (5-FU) and gemcitabine. A number of other single agents and their combinations have been assessed [6,7,8]. Novel target agents are also under investigation [9]. From a radiotherapeutic point of view, radiation field modification, radiation dose escalation, and different treatment modalities have been attempted. Since pancreas is surrounded by the duodenum and small intestines, delivering radiation doses exceeding 50.4 Gy in conventional fractionation in traditional radiation fields were nearly impossible. With intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), dose escalation with more accurate targeting and normal tissue sparing are becoming feasible.
However, even with all these attempts, treatment outcomes of locally advanced pancreatic cancer remain in the same range. Moreover, little is known about prognostic factors of the disease, especially in locally advanced cases treated with concurrent chemoradiotherapy (CCRT).
In this study, we retrospectively reviewed medical records of patients with locally advanced pancreatic cancer who were treated with definitive CCRT. Overall survival (OS) and progression-free survival (PFS) were analyzed and prognostic factors were assessed.
Materials and Methods
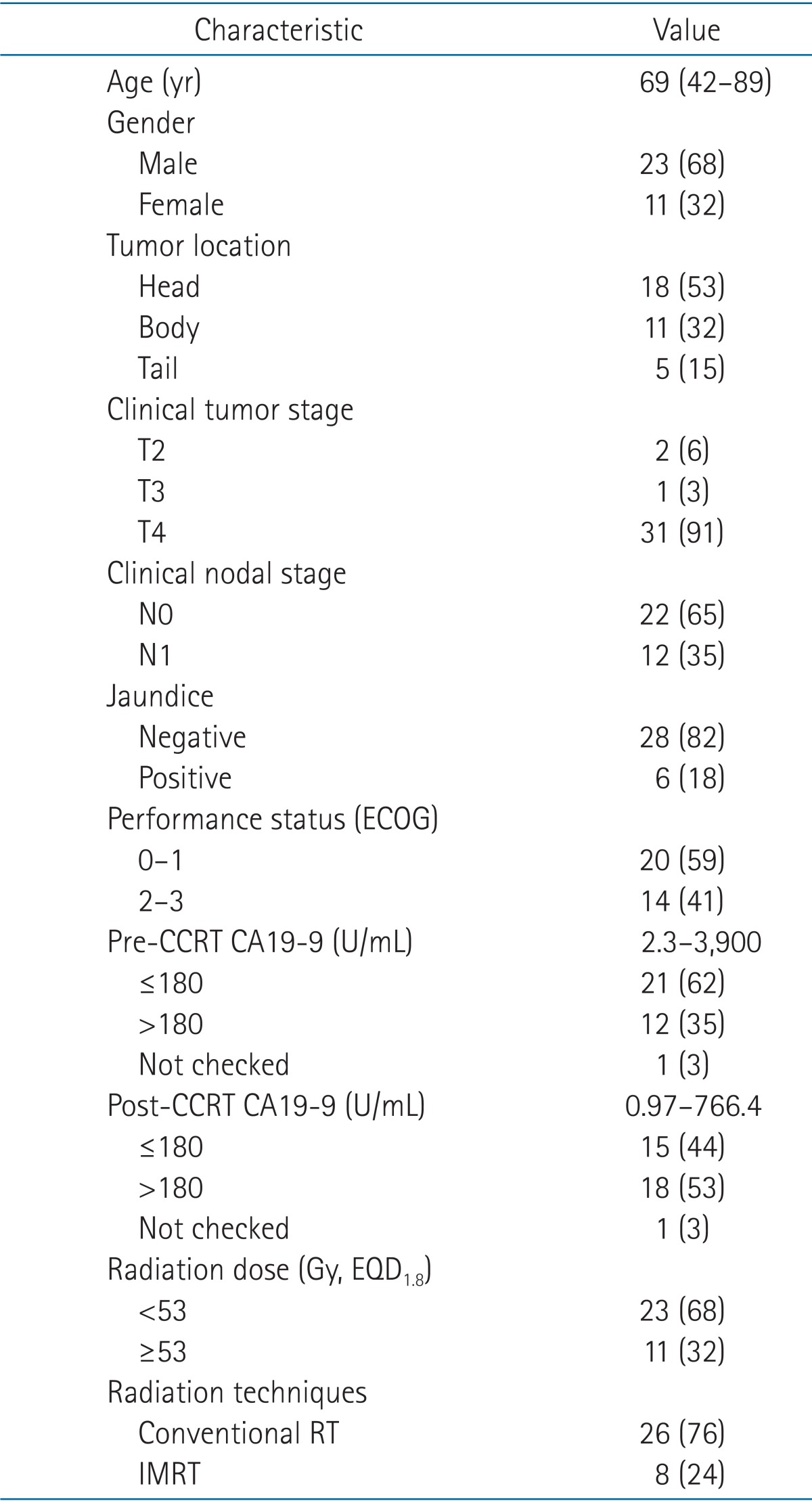
Medical records of patients diagnosed with unresectable pancreatic cancer and treated with chemoradiation, from December 2003 to December 2012, were reviewed. Among 40 patients, 2 patients who received sequential chemoradiation, 3 patients treated below 40 Gy of radiation, and one patient with multiple hepatic metastases were excluded. Conclusively, 34 patients treated with CCRT were enrolled. Of the 34 patients, 25 patients were histologically confirmed whereas remaining 9 patients failed biopsy because of the difficulties in approaching due to abutment or invasion to major vessels. Twenty-six cases met the definition on unresectable disease, according to the National Comprehensive Cancer Network guidelines: 1) ≥180 degrees of superior mesentery artery encasement, any celiac abutment, or inferior vena cava abutment; 2) unreconstructible superior mesenteric vein and/or portal occlusion; 3) aortic invasion; and 4) distant metastasis [10]. Four pancreatic tail cancers, one T2 case, and one T3 case were included, which the surgeon considered inoperable because they had splenic vessel encasement, left renal artery encasement, or left adrenal invasion. One unresectable case with T-colon invasion and one T2 case which the patient refused surgery were also included. Exclusion criteria were co-existent malignancies, history of radiotherapy, and prior surgery, such as surgical resection or surgical bypass. Approval of our Institutional Review Board was achieved.
1. Assessment
Chart review focused on patients' age, sex, the Eastern Cooperative Oncology Group (ECOG) performance status, tumor location (head, body, tail), jaundice (serum bilirubin levels), tumor histologic types, pre- and post-treatment CA19-9 levels (normal range was 0 to 37 IU/mL), and pre- and post-treatment esophagogastroduodenoscopy results. Abdomen and pelvis computed tomography (CT) scans were obtained and other radiologic studies like chest CT or FDG-positron emitting tomography scans to evaluate distant metastasis were done. Staging was done according to the American Joint Committee of Cancer (AJCC) 7th edition. If needed, endobiliary or percutaneous bile drainage was performed. After CCRT started, patients were interviewed once weekly during the whole course and routine complete blood counts and blood chemistry were examined for assessment. Patients were evaluated one month after completion of CCRT to examine the disease status and treatment outcomes. They were re-evaluated at every 3 to 6 months, thereafter.
2. Radiotherapy
Radiation was delivered at a median dose of 50.4 Gy (range, 41.4 to 55.8 Gy), once daily, 5 times per week, 1.8 to 3 Gy per fraction. Median time to complete CCRT was 5.5 weeks. Twenty-six patients were treated with conventional radiation therapy with 3-field or 4-box-field technique using high energy linear accelerator. The remaining eight patients were treated with IMRT using Tomotherapy.
Simulation was done in supine position with both arms up together and Vac-Lok (CIVCO Medical Solutions, Coralville, IA, USA) was used for immobilization. For IMRT, compression with vacuum was done to minimize respiratory movements of the abdomen. Contrast enhanced CT simulation was done and 5-mm sliced CT was obtained at least one week before CCRT began.
Gross tumor and all visible lymph nodes were delineated slice by slice. Regional lymph nodes, such as anterior and posterior pancreaticoduodenal lymph nodes, supra- and infra-pancreatic head and body nodes, SMA nodes, celiac axis nodes, hepatoduodenal ligament nodes, para-aortic nodes, aortocaval nodes, and splenic artery nodes were included. For IMRT, clinical target volume (CTV) was the regional lymph nodes plus 0.5 cm margin around the gross tumors. With concern of set-up errors and organ movements, CTV with an additional 0.5 to 1 cm margin was defined as planning target volume (PTV).
Most of the cases with conventional radiation therapy were treated with a total dose of 50.4 Gy/28 fx whereas median 50 Gy with daily 2.5 to 3 Gy/fx was delivered in cases with Tomotherapy. For patients treated with conventional radiation therapy, field size was reduced after 45 Gy to the gross tumor volumes. With Tomotherapy, simultaneous integrated boost was feasible with GTV receiving 50 to 60 Gy while PTV receiving 45 to 55 Gy in 20 to 25 fractions.
When analyzing on radiation dose, equivalent dose in 1.8 Gy per fraction (EQD1.8) was calculated, using an estimated α/β ratio of 10.
3. Chemotherapy
Chemotherapy regimen used for CCRT was 5-FU in most cases with one exception treated with FL (5-FU, leucovorin). Intravenous infusion of 5-FU was administered at a dose of 600 mg/m2, weekly, 4 to 5 times during radiotherapy or 1,000 mg/m2 for 3 days at the beginning and in the end of the radiotherapy. Gemcitabine, when given as neoadjuvant or adjuvant chemotherapy, was administered weekly at a dose of 200 mg/m2, intravenously.
Thirty-three patients received 5-FU chemotherapy and one patient received FL. Prior to CCRT, four patients (12%) received neoadjuvant chemotherapy with gemcitabine. Twenty-three patients (68%) were treated with adjuvant chemotherapy, most with gemcitabine.
4. Statistical analyses
Primary endpoint of this retrospective study was OS and PFS rates. Secondary endpoints were prognostic factors, treatment response, and treatment toxicities. OS time was counted from the date of diagnosis to death or the last follow-up date in survivors. Progression free survival time was from the date of completion of CCRT to the date of progression. Survival curves and local control rates were achieved using the Kaplan-Meier method and the differences were evaluated by log-rank test. Univariate and multivariate analyses as well as Cox proportional hazards models were used to assess prognostic factors. Treatment response was assessed by comparing abdomen CTs taken before and after radiotherapy using Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [11]. To identify the relationship between toxicities and radiation dose, Pearson correlation analysis was used. When interpreting the results, p-values less than 0.05 were considered significant. All data were computed and analyzed with SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
2. Treatment response
Treatment response was analyzable in 28 patients (82%); six patients had inadequate follow-up. Mean interval from the date of CCRT completion to treatment response evaluation was 26 days. Response evaluation was based on imaging studies taken within 3 months after completion of CCRT. None of the patients went through histologic confirmation. One patient achieved complete response and 10 patients (36%) achieved partial response. Fourteen patients (50%) had stable disease and the remaining three patients had progressive disease.
3. Overall survival and progression-free survival
With a mean follow-up of 10 months (range, 0 to 49 months), median OS was 9 months. The 1- and 2-year survival rates were 40% and 10%, respectively. Median and mean time to progression were 5 and 7 months, respectively. Median time to local progression was 9 months and distant progression was 5 months. One-year local control rate was 8%. Failure pattern in 27 evaluable patients were as follows: 2 patients were free of local or distant progression at last follow-up. Five patients had local progression and 9 patients had distant progression. Both local and distant progression occurred in 12 patients. One patient was lost to follow-up. For those who developed distant metastasis, the most common site was peritoneum (26%), followed by liver (20%), lung, bone, and lymph node.
4. Prognostic factors
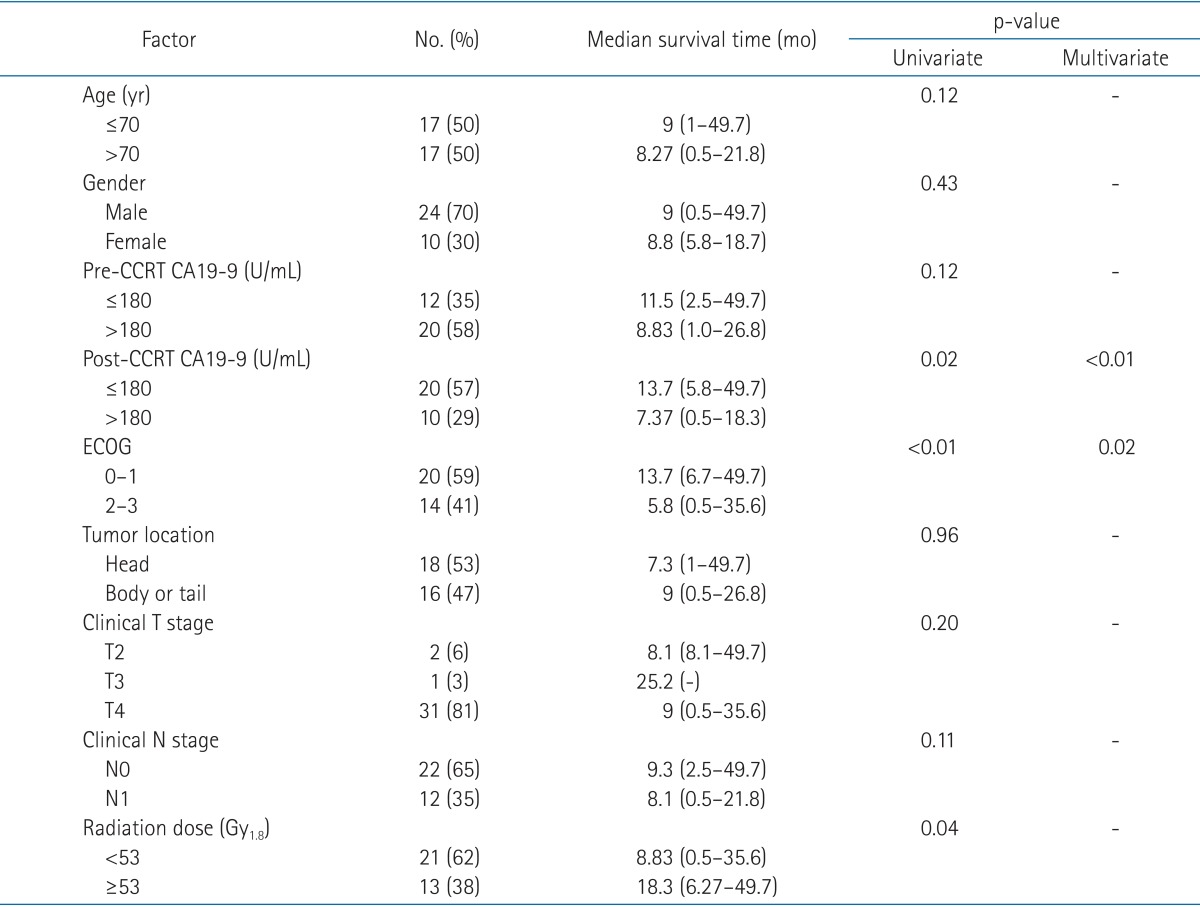
Prognostic parameters related to OS were post-CCRT CA19-9 (≥180 or <180 U/mL), ECOG status and radiation dose (≥53 or <53 Gy) according to univariate analysis (Table 2). In multivariate analysis, post-CCRT CA19-9 value below 180 U/mL and ECOG status 0 or 1 were statistically significant independent prognostic factors associated with improved OS (p < 0.01 and p = 0.02, respectively) (Figs. 1 and 2).
Prognostic factors for PFS were also assessed. Only ECOG status showed statistical significance (p = 0.02) and post-CCRT CA19-9 value showed marginal significance (p = 0.06) in univariate analysis. In multivariate analysis, ECOG 0 or 1 had statistically better PFS than ECOG 2 or 3 (p = 0.02).
One-year local control rate in radiation dose below 53 Gy was 0% compared to 13% in radiation dose ≥53 Gy. However, there was no statistically significant relationship between local control rate and radiation dose ≥53 or <53 Gy (p = 0.47).
5. Toxicities
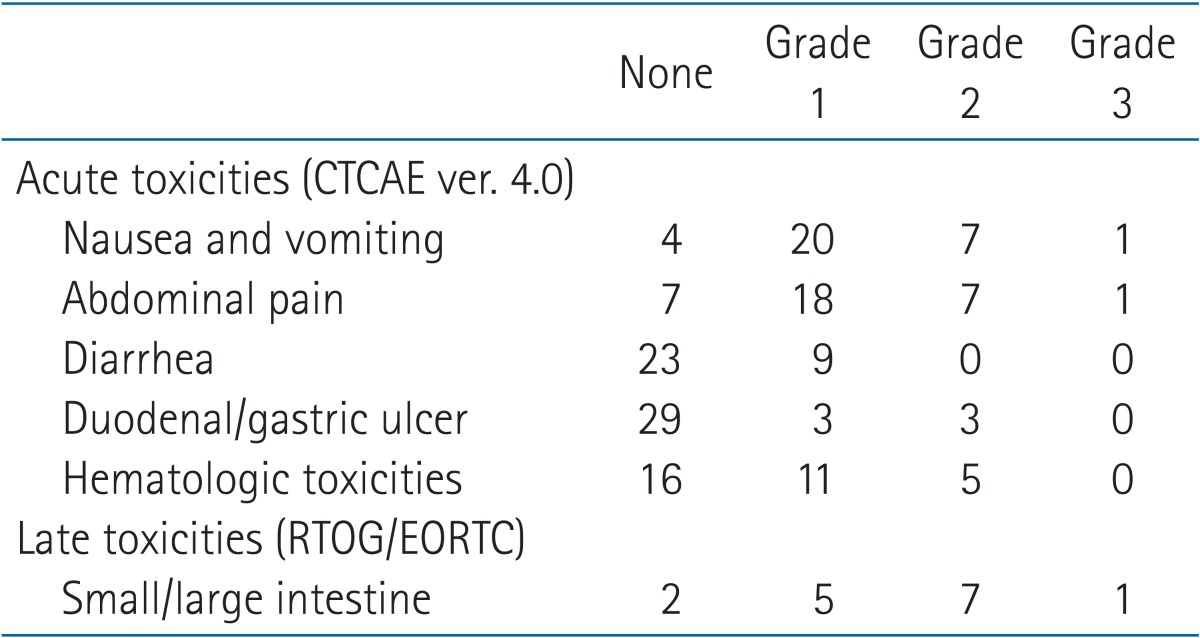
Acute toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. All 34 patients were assessed for acute toxicities (Table 3). One patient suffered from grade 3 abdominal pain and thrombocytopenia with neutropenia. This patient was treated with neoadjuvant chemotherapy with 6 cycles of erlotinib and gemcitabine followed by CCRT with epirubicin, cisplatin, and oral UFT (ECU). One patient experienced grade 3 nausea. Three patients experienced duodenal or gastric ulcer bleeding within 2 months after CCRT completion and were managed by proton pump inhibitor. No treatment related life threatening events or death occurred. Late toxicities, which occurred 3 months after CCRT, were graded according to RTOG/EORTC Radiation Toxicity Grading [12]. Only 14 patients (40%) were evaluable for late complications because of follow-up loss and early deaths. One patient with duodenal 2nd portion obstruction required stent insertion. Correlation between toxicities and radiation dose or treatment modalities did not reach statistical significance.
Discussion and Conclusion
Prognosis of unresectable, locally advanced pancreatic cancer is depressing and not many therapeutic advances have been forthcoming. In 1980, the Gastrointestinal Tumor Study Group (GITSG) reported better treatment outcomes of locally advanced pancreatic cancer with CCRT compared to radiotherapy alone with median survival times of 10 and 6 months, respectively [3]. Several other randomized trials comparing single modality therapy, either chemotherapy alone or radiotherapy alone, to CCRT was performed: a median survival of 8.3 to 11.4 months in combined-modality arms and 5.5 to 7.4 months in single-modality arms were reported [13,14]. In our study, the median OS was 9 months. Although our survival outcome is in the lower range of the reported data, grade 3 or higher late (7%) toxicities were acceptable and there were no fatal complications. In one prospective study, severe late toxicities (26%) occurred and there was 1 treatment related death although they showed improved OS with high dose radiotherapy [15].
To improve local control rates of unresectable pancreatic cancer, investigators are trying to find ways to deliver higher radiation doses. The GITSG in 1981 compared 40 Gy and 60 Gy plus 5-FU, but the results did not yield significant survival differences. The authors used anterior-posterior opposing radiation beams [3]. Murphy et al. [16] confirmed that radiation fields covering only the gross tumors and omitting prophylactic lymph nodes do not cause marginal failures. In their study, three-dimensional conformal radiotherapy technique was adopted and even with full-dose gemcitabine, they reported reduced toxicities. Chang et al. [15] reported on high-dose helical tomotherapy with concurrent chemotherapy and demonstrated significantly enhanced local control and long-term survival. Median radiation dose was 58.4 Gy. Golden et al. [17] demonstrated a superior OS in patients who received radiation dose exceeding 54 Gy. However, our study was incompetent in proving improved local control rate by delivering higher radiation dose. This was likely due to our targets in IMRT cases included regional lymph nodes, unlike other studies that included only gross tumors. Therefore, satisfying dose escalation could not be done. Perhaps with smaller targets confined to gross tumor volumes, we would be able to deliver higher doses and demonstrate an improved local control like the other two mentioned studies. Recent trials are improving on accurate targeting and dose escalation with IMRT, SBRT, or proton therapy, expecting on improvements in both local control rates and OS rates [18,19,20]. Although firm evidence with phase III trial is lacking, some of these studies suggest encouraging outcomes.
In this study, 44% had local and distant failure, 37% had distant metastasis, and 19% had local progression. High rates of distant failure imply that using more effective systemic chemotherapy in a CCRT regimen could improve treatment outcomes. FFCD-SFRO in 2001 reported improved survival rates by treating locally advanced pancreatic cancer with gemcitabine alone [21]. However, Crane et al. [22] compared the toxicity of CCRT with gemcitabine to 5-FU and demonstrated significantly higher severe toxicity in gemcitabine group. Several other groups analyzed on gemcitabine-based CCRT and reported superior or at least similar survival rates over 5-FU. Among these, some studies reduced toxicities by reducing radiation field size [16,23,24,25]. Upon these attempts, cautious anticipation can be made on reducing distant failures with a more effective systemic chemotherapy.
Since the treatment outcome of unresectable pancreatic cancer is dismal, not much data are available to address prognostic factors of the disease. Several studies with different treatments and patient groups have shown a relationship between CA19-9 and treatment response [26,27,28]. Yoo et al. [29] reported pre-treatment and post-treatment CA19-9 levels were significantly related to OS in univariate analysis. In multivariate analysis, only pre-treatment CA19-9 was statistically significant. Their cut-off values for pre-treatment CA19-9 was 400 U/mL and for post-treatment CA19-9 was 200 U/mL. Micke et al. [30] also reported that CA19-9 was predictive for prognosis, response, and recurrence. Both pre- and post-treatment CA19-9 values were statistically significant. Their cut-off values were the median values; 420 U/mL for pre-treatment CA19-9 and 293 U/mL for post-treatment CA19-9. Montgomery et al. [31] published on post-resection CA19-9 values in adenocarcinoma of the pancreas and postoperative CA19-9 <180 U/mL had similar disease-free and median survival compared to the patients who reached normal values after resection. Our study also demonstrated improved OS with lower post-treatment CA19-9 values. Cut-off value used for analysis was the median of all measured post-treatment serum CA19-9 levels. Improved OS was observed in patients whose post-CCRT CA19-9 values were below 180 U/mL.
There are several limitations in this study. First, this is a retrospective study and selection bias is inevitable. Toxicities were recorded upon physicians' subjective judgments and this also could have contributed to inaccurate data collection. Moreover, our sample size is relatively small. To keep the cohort homogeneous, only a small number of patients were chosen. All of the patients were treated with radiation dose ≥40 Gy and treated with 5-FU, except for one case. In the current study, Lewis (Le) blood group antigen was not evaluated in the patients diagnosed with pancreatic cancer. In patients with Lea-b- phenotypes, CA19-9 elevation is not detected and this occurs in 5% of the population [32].
Despite the limitations, treatment outcomes in our study are in the range of other published reports on the treatment of locally advanced pancreas cancer. Along with the results, we assume post-treatment serum CA19-9 levels and performance status have an impact on the prognosis of the disease.
In conclusion, this study shows comparable outcomes of locally advanced pancreatic cancer treated with CCRT as other studies with acceptable toxicities. The prognostic factors related to survival rates from this study were performance status and post-treatment serum CA19-9 levels. Since the results of CCRT with conventional fractionation and extant chemotherapy remains in a similar range for decades, the emerging and encouraging data on SBRT or proton therapy could be a treatment alternative in the future.
Notes
No potential conflict of interest relevant to this article was reported.