Postoperative radiation therapy following the incomplete resection of a non-small cell lung cancer
Article information
Abstract
Purpose
To review the results of postoperative radiation therapy (PORT) for residual non-small cell lung cancer (NSCLC) following surgical resection and evaluate multiple clinicopathologic prognostic factors.
Materials and Methods
A total of 58 patients, who completed scheduled PORT for positive resection margin, among 658 patients treated with PORT from January 2001 to November 2011 were retrospectively analyzed. Radiation therapy was started at 4 to 6 weeks after surgery. Chemotherapy was also administered to 35 patients, either sequentially or concurrently with PORT.
Results
The median age of patients was 63 years (range, 40 to 82 years). The postoperative pathological stage I NSCLC was diagnosed in 10 (17.2%), stage II in 18 (31.0%), and stage III in 30 patients (51.7%). Squamous cell carcinoma was identified in 43, adenocarcinoma in 10, large cell in 1, others in 4 patients. Microscopic residual disease (R1) was diagnosed in 55 patients (94.8%), and the remaining three patients were diagnosed with gross residual disease (R2). The median dose of PORT was 59.4 Gy (range, 50.0 to 64.8 Gy). Chemotherapy was administered to 35 patients (60%), and the median follow-up time was 22.0 months (range, 6.0 to 84.0 months). The 3-year locoregional relapse-free survival and distant metastasis-free survival rates were 82.1% and 52.9%, respectively. The median overall survival was 23.8 months (range, 6.0 to 84.1 months), and the 3-year overall survival rate was 58.2%. Chemotherapy did not influence the failure pattern or survival outcome.
Conclusion
PORT is an effective modality for improving local tumor control in incompletely resected NSCLC patients. Major failure pattern was distant metastasis despite chemotherapy.
Introduction
Complete surgical resection is the treatment of choice for curing locally advanced non-small cell lung cancer (NSCLC). However, 1% to 17% of surgical resections for NSCLC are reportedly incomplete [1,2]. Incomplete resection is associated with increased local recurrence and negatively impacts on survival [2,3,4,5]. Postoperative radiation therapy (PORT) is essential for improving local tumor control in patients with incompletely resected NSCLC, and the National Comprehensive Cancer Network guidelines recommend combination chemoradiation therapy for incompletely resected, locally advanced NSCLC. The type of combination adjuvant treatment-sequential or concurrent chemoradiation-is determined by the extent of residual disease, age and performance of patient, and pathological stage. Concurrent chemoradiation therapy (CCRT) may result in better local tumor control, but it can also result in additional treatment-related morbidity and poor tolerance in adjuvant settings following major surgery [6].
In this retrospective analysis, we reviewed the treatment outcomes of PORT for incompletely resected NSCLC and also evaluated multiple clinicopathologic prognostic factors.
Materials and Methods
Between January 2001 and November 2011, 658 patients with resected NSCLC received PORT at our institution. The medical records of these cases were fully reviewed, and 64 patients (9.7%) had positive surgical margins noted in their pathological reports. Six patients were excluded because they received incomplete PORT during the early course of disease (progression of pneumonia during treatment in 5, aggravation of acute renal failure which was unrelated with lung cancer treatment in 1). A total of 58 patients were then retrospectively reviewed, and this study was approved by the Institutional Review Board of our hospital.
All patients had pathologically confirmed NSCLC. R1 resection was defined as residual microscopic tumor on the surgical margins, and R2 resection was defined as residual macroscopic tumor determined by surgical findings or pathological reports. Pathological stage was determined according to the 7th edition of the American Joint Committee on Cancer. All patients underwent video-assisted thoracoscopic surgery-either thoracoscopic or open thoracotomy-with lobectomy, pneumonectomy, and sleeve resection. Mediastinal lymphadenectomy or systematic mediastinal lymph node sampling was also performed on all patients.
Computed tomography (CT)-simulation for PORT was done in all patients. The patients were placed in the supine position with arm-side, and immobilized using a pillow and vacuum mold. The target volume was delineated, which included the surgical stump, area of residual disease, ipsilateral hilum, and also covered both sides of the mediastinum, and the ipsilateral supraclavicular lymph nodes in patient with pN2. This area was defined as the clinical target volume (CTV). The planning target volume included 1-cm margins from the CTV. Boost radiation following initial treatment was only delivered to areas with residual disease after 50.4 Gy. Chemotherapy was administered according to nodal status. In most patients who received sequential chemoradiotherapy (CRT), radiation was delivered before or after systemic chemotherapy with monthly vinorebine + cisplatin. For CCRT, weekly docetaxel or paclitaxel + cisplatin was administered. Thirty-five of 58 patients (60.3%) received chemotherapy.
Patients were evaluated every 3 months for 2 years after treatment, and every 6 months thereafter. Chest X-rays or chest CT was performed as part of the regular follow-up examinations. We used the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) to assess side effects.
Overall survival (OS), locoregional relapse-free survival (LRFS), and distant metastasis-free survival (DMFS) were calculated using the Kaplan-Meier method. OS was defined from the day of surgical resection to the time of death or last follow-up examination. Local relapse was defined as any recurrence at the surgical resection margin, and regional relapse was defined as mediastinal recurrence (except at the surgical margins). Distant metastasis was defined as recurrence at any other organ or lung lobe. The log-rank method was used to compare sequential CRT with CCRT in terms of OS, LRFS, and DMFS. SPSS ver. 21.0 (IBM, Armonk, NY, USA) was used to perform the statistical analysis.
Results
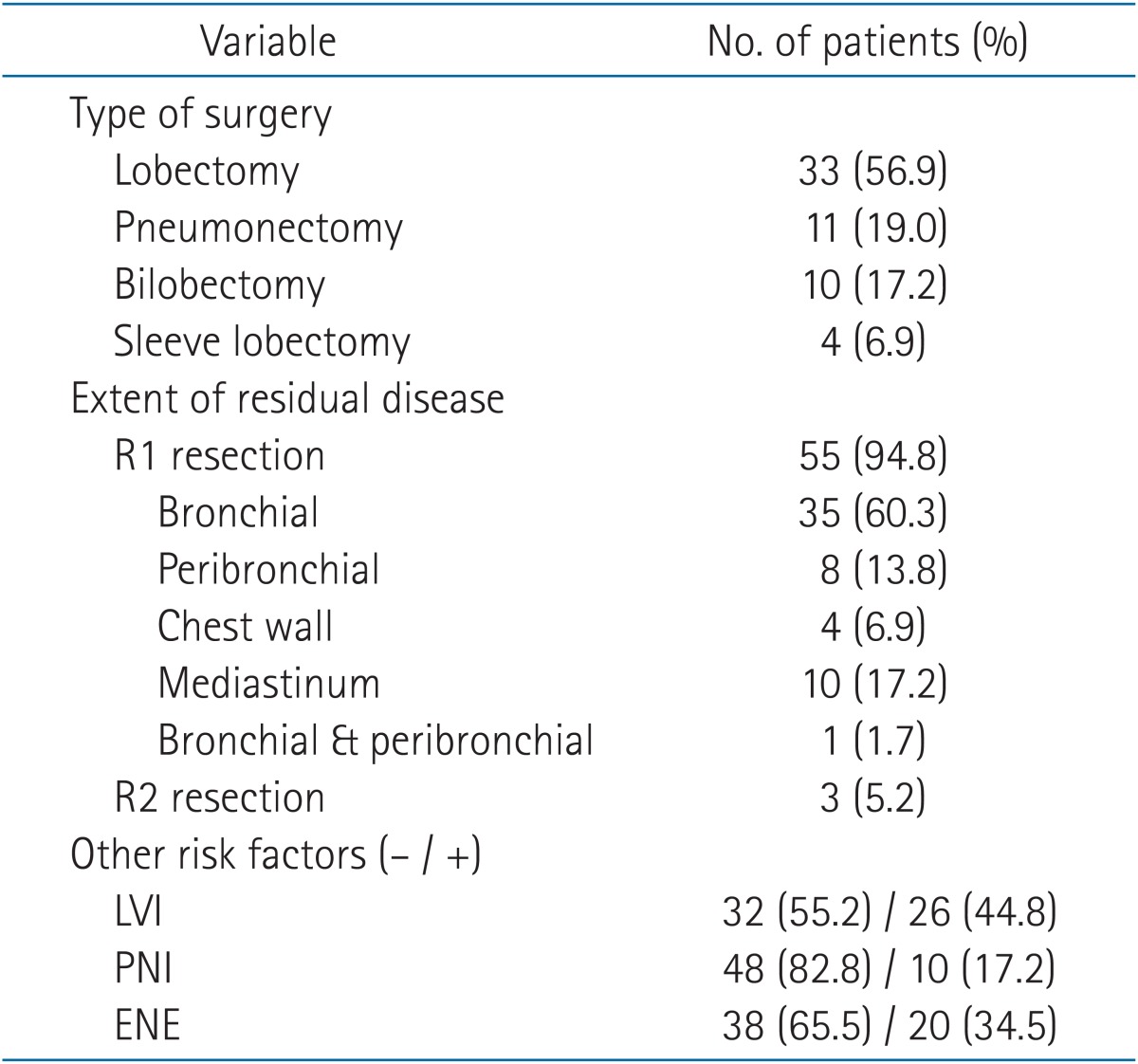
Fifty-six patients (96.5%) were male, and the median age was 63 years (range, 40 to 82 years). Squamous cell carcinoma was identified in 43 patients (74.1%; the most common histological type), and 10 patients (17.2%) were diagnosed with adenocarcinoma. Sixteen patients (27.6%) received PORT due to positive resection margins without lymph node metastasis. Most patients were classified as stage II or IIIA (74.1%). The patient characteristics are listed in Table 1 and the surgical characteristics are summarized in Table 2. Most incomplete resections were R1 (55 patients), and the most common site of residual disease was the bronchial resection margins (60.3%).
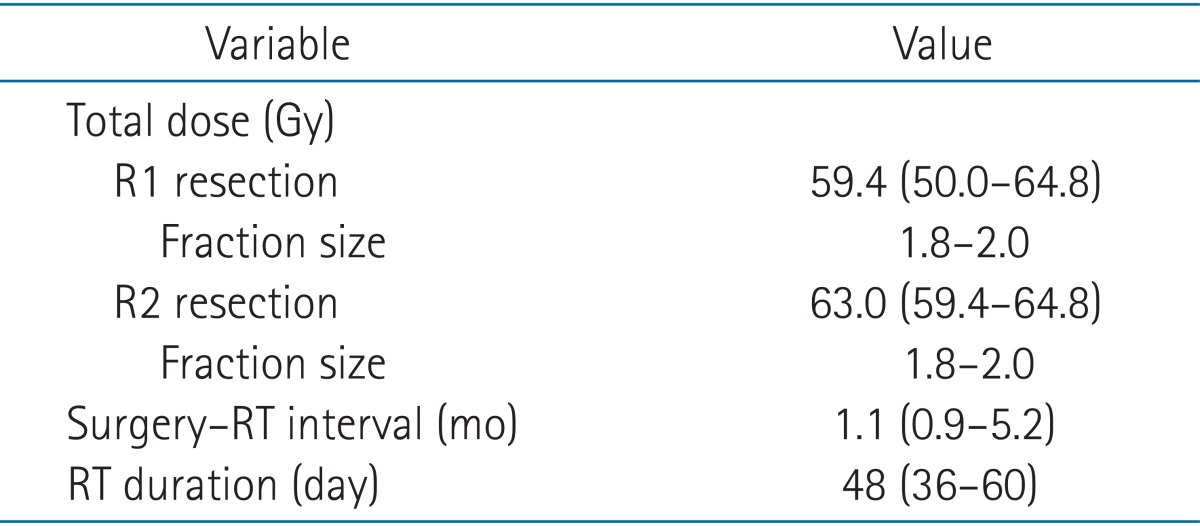
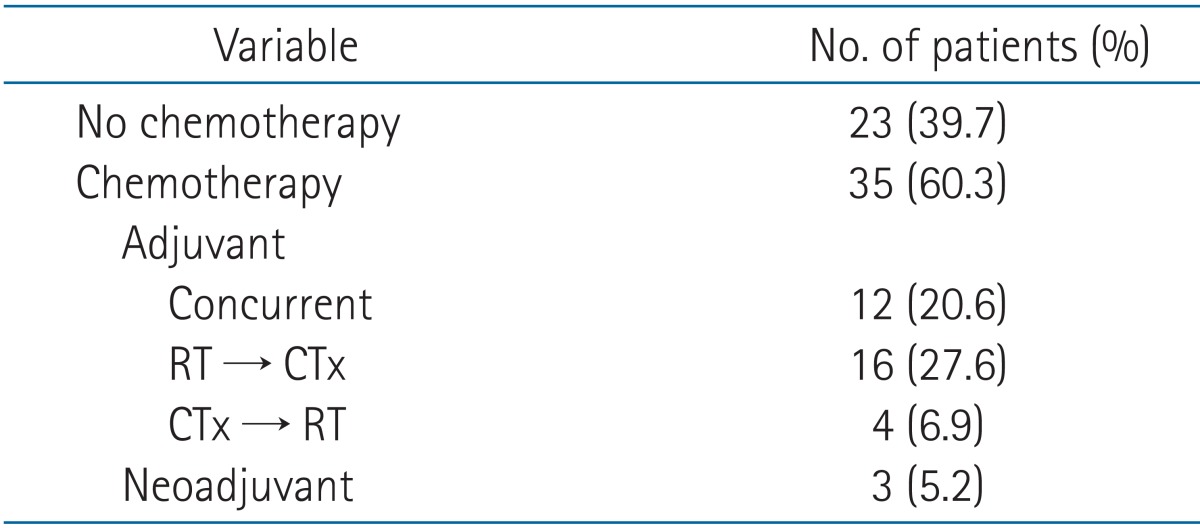
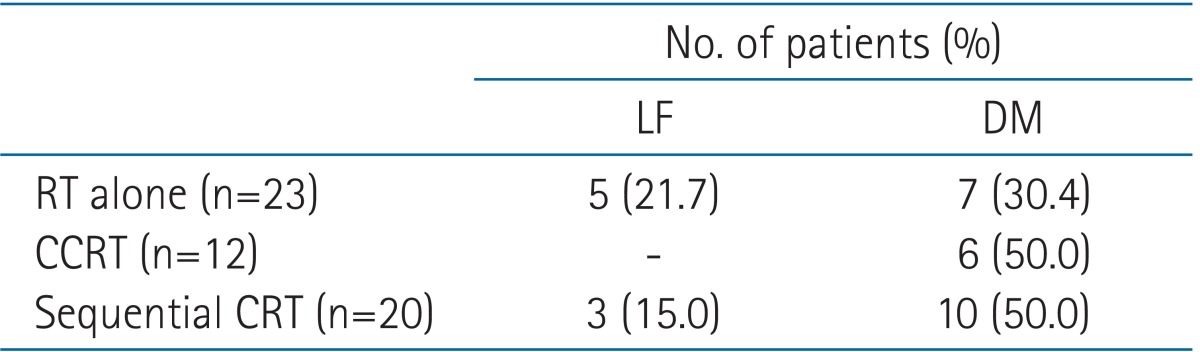
All patients were treated with external beam radiation therapy. The median total dose administered to R1 resections was 59.4 Gy (range, 50.0 to 64.8 Gy), and 63.0 Gy (range, 59.4 to 64.8 Gy) was administered to R2 resections. Time between surgery and the start of radiation therapy ranged between 0.9 to 5.2 months. Patients with adjuvant chemotherapy followed by RT had 4 months more interval between surgery and the start of RT (Table 3). Thirty-five patients received chemotherapy, including 3 patients who received chemotherapy before surgery. Twelve patients (20.6%) were treated with adjuvant CCRT, and 20 patients (34.5%) received sequential CRT (RT followed by chemotherapy or chemotherapy followed by RT) (Table 4).
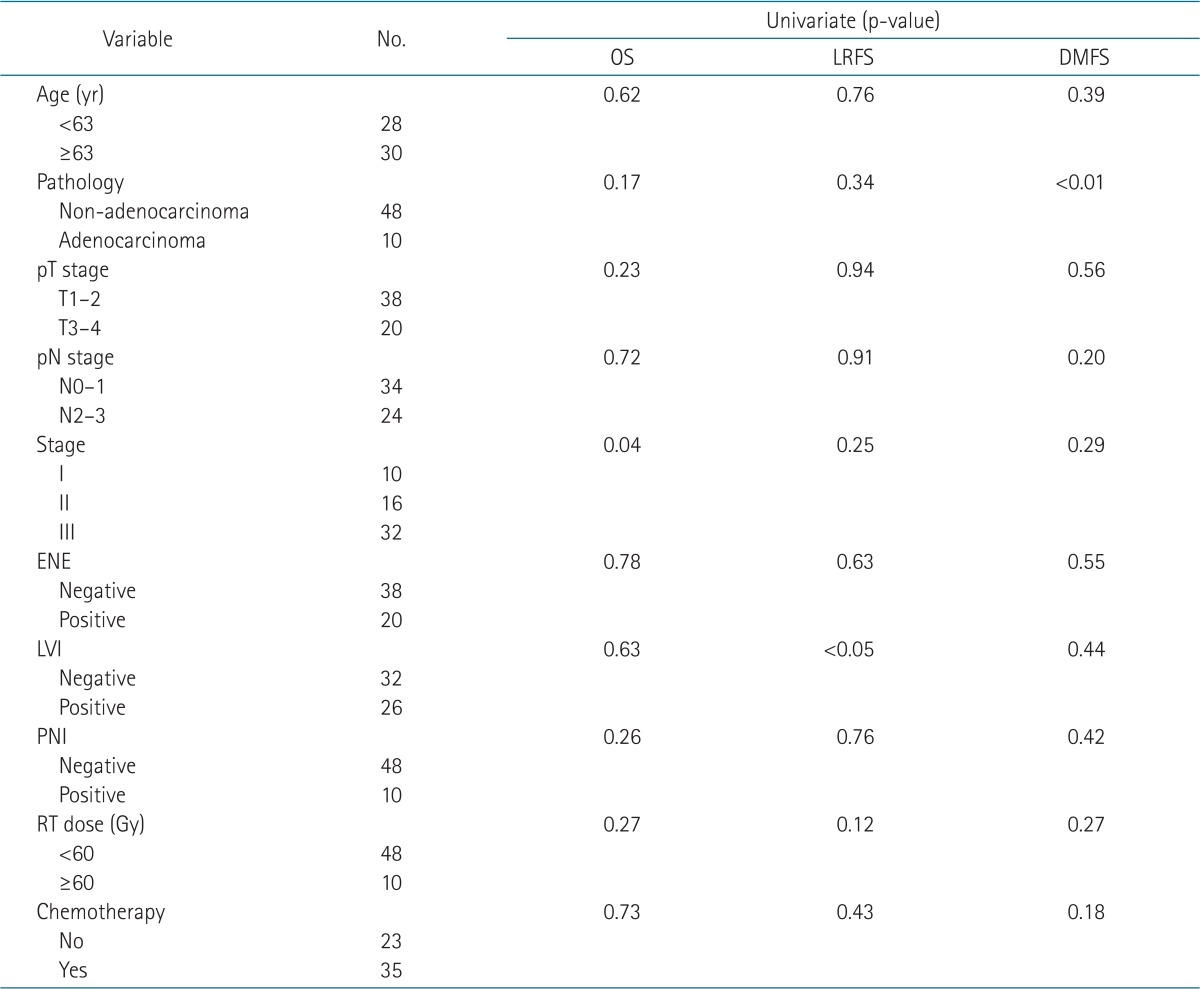
The median follow-up period was 22.0 months (range, 6.0 to 84.0 months). The median overall survival was 23.8 months (range, 6.0 to 84.1 months), and the 3-year OS rate was 49.3% (Fig. 1). Eight patients (13.8%) developed locoregional recurrence, and the resection margins were the most common site (7 of 8 patients). Twenty-three patients (39.7%) were diagnosed with distant metastasis. The 3-year LRFS and DMFS rates were 82.1% and 52.9%, respectively (Fig. 2). According to univariate analysis, stage, lymphovascular invasion, and pathological type were significant prognostic factors associated with OS, LRFS, and DMFS, respectively (Table 5).
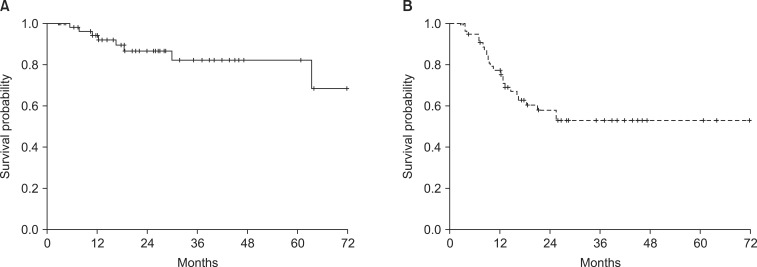
(A) Locoregional relapse-free survival rate and (B) distant metastasis-free survival rate of all patients.
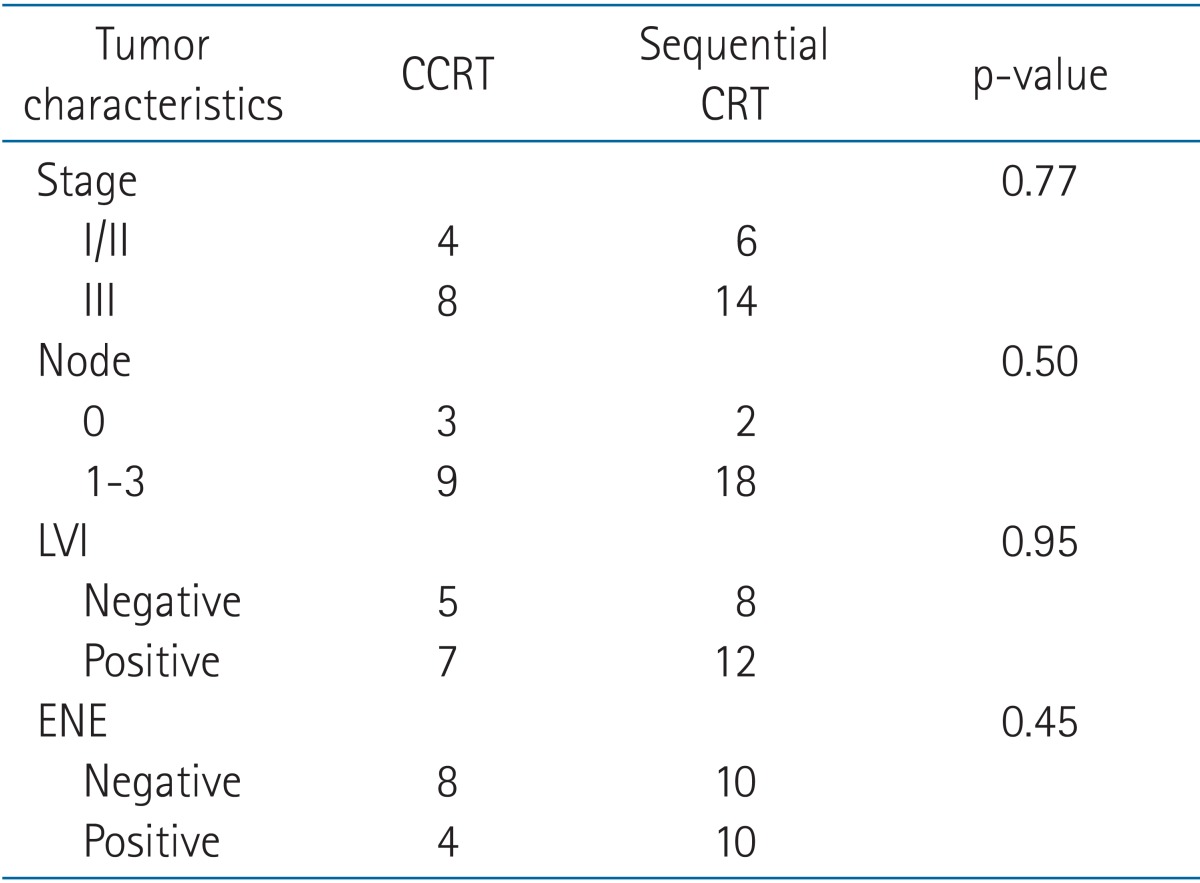
There were no differences between the CCRT and sequential CRT groups in terms of tumor characteristics (Table 6). Both the CCRT and sequential CRT groups demonstrated better local control compared with the RT-alone group (Table 7). The CCRT group demonstrated relatively superior local tumor control than sequential CRT group, but this finding was not statistically significant (p = 0.23) (Fig. 3). Distant metastasis was a major pattern of failure detected in both groups despite combination chemotherapy. The distant failure rate was 50% in both the CCRT and sequential CRT groups. No patient developed severe acute or late toxicity greater than grade 3. Twenty-seven patients (46.5%) demonstrated acute grade 2 toxicity, and the most common symptom was esophagitis. Late grade 2 lung toxicity was only observed in 2 patients.
Discussion and Conclusion
Incomplete resection might be a negative prognostic factor for local tumor control and survival in patients with locally advanced NSCLC. Wind et al. [7] previously reported 1-year and 5-year OS rates of 20% to 50% and 0% to 20%, respectively, suggesting that residual disease in the peribronchial resection margins is associated with mediastinal lymph node metastasis. Riquet et al. [3] reported poor survival in patients with incomplete resection in comparison with complete resection (5-year OS: 20% vs. 46%, respectively; p < 0.001).
Some studies report that administering PORT to patients with incomplete resection margins improves treatment outcomes. Ghiribelli et al. [8] reported 3-year OS and local control rates of 40% and 27.5%, respectively, in incompletely resected NSCLC patients who received PORT without chemotherapy. Ohguri et al. [2] also reported that 5-year OS, local control, disease-free, and DMFS rates were 56%, 63%, 37%, and 49%, respectively, in patients with incomplete resection margins who received PORT. The study of Ohguri et al. [2] reported a high local tumor control rate, although 44% of their reviewed patients had R2 resection margins and the most common failure pattern was distant metastasis. These authors emphasized the necessity of effective systemic control.
In this retrospective study, PORT with chemotherapy resulted in high local tumor control (3-year LRFS, 82.9%) in patients with incompletely resected NSCLC. This indicates better local tumor control than previously reported outcomes due to the high proportion of patients with R1 resection margins and combination chemoradiation. The target volume in all of our patients was defined for elective nodal irradiation (ENI), although the extent varied depending on the extent of the involved lymph nodes. This is probably why isolated regional relapse was observed in only one case in our study. Olszyna-Serementa et al. [9] previously compared ENI + PORT and omitting ENI for the treatment of incompletely resected tumors. No statistical difference was found in the OS or local control between the two groups in that report, but higher local recurrence (7.5% vs 22.5%, respectively) and isolated nodal failure (7.5% vs 13.5%) were noted in patients who did not receive ENI.
Most previous studies have reported distant metastasis from incompletely resected NSCLC as a major failure pattern in this setting. According to univariate analysis of our study, adenocarcinoma is the only significant risk factor for DMFS. Ohguri et al. [2] reported that adenocarcinoma is a significant predictor of distant metastasis in patients with incompletely resected NSCLC who received PORT (p = 0.03). These data suggested that effective systemic control is essential for improving survival because local recurrence and residual disease can be controlled using adjuvant radiation therapy. According to our current study results, combination adjuvant chemoradiotherapy demonstrates good local tumor control of incompletely resected NSCLC, but distant metastasis is still a major failure pattern. The results were similar regardless of the type of combination chemoradiation used (i.e., sequential CRT or CCRT).
There have been no earlier studies comparing postoperative concurrent and sequential CRT. The differences between postoperative concurrent and sequential CRT could be inferred from the Cochrane database, although this is the meta-analysis of definitive setting. According to the Cochrane database which compares definitive CCRT and sequential chemoradiotherapy for treating NSCLC, CCRT demonstrates better treatment outcomes in local tumor control and OS. However, there are higher rates of acute grade 3 or greater esophagitis in patients who receive concurrent chemoradiotherapy in comparison with sequential combination chemoradiotherapy (relative risk [RR], 4.96; 95% confidence interval [CI], 2.17 to 11.37), and higher rates of grade 4 or greater neutropenia in the concurrent group (RR, 1.18; 95% CI, 0.90 to 1.55) [10].
The results of our study show that CCRT can provide relatively better local tumor control than sequential CRT, but that sequential CRT also demonstrates good local tumor control. Distant metastasis did not decline following the administration of adjuvant chemotherapy in CCRT and sequential CRT groups, and sequential CRT can be safely applied to patients with poor performance, old age, or multiple morbidities (whereas CCRT cannot be applied in these situations). However, our study is limited by its retrospective design and the relatively small number of patients analyzed.
Administering PORT to incompletely resected NSCLC is an effective modality for improving local tumor control. Combination chemoradiotherapy can be better than PORT alone for local tumor control, but CCRT may be more effective than sequential CRT. Distant metastasis is still a major failure pattern in patients with incompletely resected NSCLC, even if combination chemoradiation is administered.
Acknowledgments
This study was supported by a grant (2013-472) from the Asan Institute for Life Sciences, Seoul, Korea and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2013R1A1A2011346).
Notes
No potential conflict of interest relevant to this article was reported.