Contemporary treatment with radiosurgery for spine metastasis and spinal cord compression in 2015
Article information
Abstract
With the progress of image-guided localization, body immobilization system, and computerized delivery of intensity-modulated radiation delivery, it became possible to perform spine radiosurgery. The next question is how to translate the high technology treatment to the clinical application. Clinical trials have been performed to demonstrate the feasibility of spine radiosurgery and efficacy of the treatment in the setting of spine metastasis, leading to the randomized trials by a cooperative group. Radiosurgery has also demonstrated its efficacy to decompress the spinal cord compression in selected group of patients. The experience indicates that spine radiosurgery has a potential to change the clinical practice in the management of spine metastasis and spinal cord compression.
Introduction
Spinal metastasis is a common complication of most cancers, and when left untreated, it can progress to spinal cord compression. The treatment of spinal metastases has been palliative, with the goals of providing pain relief and possible improvement in neurologic function. This was based on the assumption that the cases of spine metastases represent terminal prognosis. However, recent advances of targeted therapy improved systemic tumor control and overall survival for patients with certain tumors and particularly with limited metastatic spread, known as oligometastases [1]. These advances have increased the need to provide more effective tumor control with durable palliation for the patients with spine metastases. Indeed, the median overall survival of patients with limited spine metastases is approximately 12 months, ranging 6 months to several years depending on the primary tumor histology [2]. Although spinal metastasis classifies as stage IV disease, the overall survival is certainly the same or longer than certain primary tumors, such as pancreatic cancers or glioblastoma multiforme. Thus, more aggressive treatment is warranted to improve the tumor control and quality of life. During the past decade, we have witnessed the development of a plethora of new technologies that have impacted the outcomes of patients with spine tumors or oligometastases. Most notable may be the evolution and integration of spinal stereotactic radiosurgery (SRS), which has demonstrated improved local tumor control compared to conventional external beam radiation (cEBRT).
Physics Aspect of Radiosurgery
The physical hallmark of radiosurgery is rapid radiation dose fall-off outside the target. It is usually represented by the conformality index. When the target is round or elliptical as in most of the cerebral metastatic lesions, the conformality index is useful. However, when the target lesion shape is irregular (like spine lesions) and radiation intensity modulation is used for planning, the distance from the point of high radiation dose (usually the prescription isodose line) to another point of lower radiation dose, 90% to 50% isodose lines for example, will be much more practical. It is usually 2-5 mm between these two isodose lines. This unique physical property allows radiosurgery to deliver a much higher radiation dose than the conventional radiotherapy. In order to safely deliver the higher dose to the target, precise and accurate targeting is required to ensure that the radiosurgical beam with rapid dose fall-off be applied precisely over the target. Since the beam is so finely focused with a rapid dose gradient, proper positioning of patient and accurate targeting are tightly coupled together for carrying out the procedure of radiosurgery for spine and spinal cord tumors.
The merit of spine radiosurgery is that the spine is well visualized using X-ray image-guidance, and thus the spine itself can be used as a fiducial for volume targeting. This makes it easier to overcome the obstacle of immobilization and patient positioning. Several imaging studies are available for improved visualization of the spine and target tumors in relation to the spinal cord. The advent of intensity modulated radiation delivery, micro-multileaf collimators, and the use of dynamic arcs increased the dosimetric precision of dose delivered to the tumor and avoidance of dose to the adjacent spinal cord. Progress in all these elements has made the application of radiosurgery to the spine feasible and spine radiosurgery has become a prototype for extra-cranial radiosurgery [3]. Radiosurgery of body sites is officially called stereotactic body radiation therapy (SBRT) by the American Society of Radiation Oncology (ASTRO) and American College of Radiology (ACR).
Biological Aspect of Radiosurgery
Radiosurgery uses high doses of radiation in a single or a few fractions in contrast to cEBRT, but it is not well understood whether a single high dose of radiosurgery has a different mechanism of cell killing mechanism compared to the conventional fractionated radiotherapy. Understanding of radiobiological effect of radiosurgery has recently been evolving. Most radiobiological studies have been performed with conventional radiation therapy with 1.8-2 Gy per fraction. Mammalian cells die through different molecular and cellular mechanisms following exposure to ionizing radiation. Depending on the cell type, irradiated cells primarily undergo reproductive cell death, also known as mitotic death. This is the predominant mode of cell death in the majority of human tumors following irradiation. Another mode of cell death is known as interphase death mostly in the form of apoptosis. It can occur in normal tissues and in some tumors particularly during the acute phase of radiation response. Some stem cells of self-renewal normal tissues, such as hematopoietic and the intestinal crypt cells, undergo apoptosis following a moderate dose of radiation. The late tissue response to radiation may also be a result of terminal growth inhibition of either self-renewing or differentiating and metabolically active cells.
Recent radiobiological evidence suggests that tumor response to radiation regulated by intestinal endothelial cell apoptosis is seen at a single dose of less than 10 Gy, while higher doses of 18-20 Gy causes death of tumor cells independent of endothelial apoptosis [4]. This supports, at least in part, an alternative pathway of molecular events within the cell following a high single dose of radiosurgery. Preliminary radiobiological experiments suggest that the initial molecular events may include rapid up-regulation of gene transcription of inflammatory cytokines, angiogenic factors, and transcription activators, and various gene products that may regulate the DNA damage fixation or the repair [5]. Taken together, these findings suggest a rather well-orchestrated cascade of cell death and repair mechanism following radiosurgical treatment. A better radiobiological understanding will help selection of radiosurgical dose, fractionation pattern, and potential combination of radiosurgery with other treatment modalities.
Application to Spine
1. Positioning and immobilization
The first step in developing spine radiosurgery is to determine targeting accuracy. Many phantom studies have demonstrated the clinical feasibility of achieving submillimeter accuracy [6]. The first human study of spine radiosurgery, however, showed the targeting accuracy and reproducibility of patient positioning were within 1.5 mm [7]. The dose fall off from the 90% to 50% isodose line was less than 5 mm towards the spinal cord. The overall procedure of spine radiosurgery includes patient positioning and immobilization, image acquisition, tumor and normal tissue delineation, radiation treatment planning, repositioning and treatment delivery under image-guidance.
The next step of applying radiosurgery to the spine is positioning and immobilization and are involuntary organ motions. There is no perfect method of immobilization. It seems important to make the patient feel stable and comfortable at the treatment position with minimal pressures at any body part. Experience with the spine radiosurgery has shown that breathing-related organ motion exists, but it does not significantly affect treatment outcome. There also potential voluntary or involuntary motions secondary to swallowing, pulsation or coughing. Some of these motions can be reduced with proper premedication. Important to the practice is to minimize any movements that can be controlled by premedication. One notable example is random movement due to spine pain, which can be minimized by proper pain medication before the procedure. Conscious sedation may further reduce most of the voluntary movements.
Although the concept of spine radiosurgery had been conceived even 50 years before the start of cranial radiosurgery, clinical application was limited mainly because of the difficulty with immobilization. In an attempt to address this issue, invasive procedure initially by anchoring hardware to the cervical spine and skull, and recently anchoring of the stereotactic frame to the lumbar spinous process under general anesthesia have been introduced [8]. Other less invasive immobilization devices used a body frame with contour mold fixation [9]. Most recently, a frameless and non-invasive positioning method for spine radiosurgery by Ryu et al. [7] and Yin et al. [10] has been developed. Most institutions now use the newer type of positioning devices for spine radiosurgery. It is important to use a method with which the patient feels comfortable while in the treatment position.
2. Target delineation
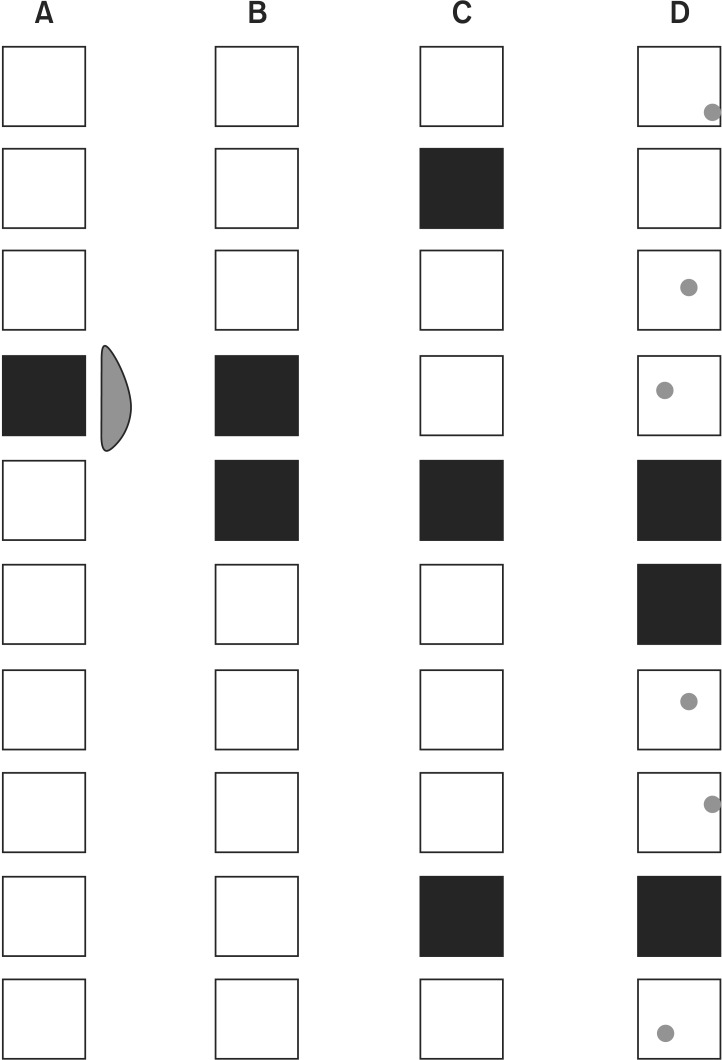
Each vertebral bone is composed of one compact bone marrow with a potential anatomic barrier at the pedicle area during the ossification phase. Although imaging studies sometimes show the gross disease within the vertebral body, it may not represent the full extent of gross tumor volume, i.e., known as GTV. Therefore, the combined volume based on the concept of clinical tumor volume (CTV) incorporating GTV should be the target for spine radiosurgery, which ultimately is the entire vertebral body of the involved spine. The method of target delineation adopted in the RTOG 0631 trial is illustrated in Fig. 1. There can be many different scenarios of spine involvement. In general, spine metastasis is modeled on three different scenarios: the most common case involves the vertebral body alone (Fig. 1A), involvement of the vertebral body extending to the pedicles (Fig. 1B), or involvement of the dorsal elements, such as spinous process and lamina (Fig. 1C). Lesions more extensively involving the vertebral body and pedicles are either treated with a generous margin (dotted line) or treated including both anterior and posterior elements (solid line) as shown in Fig. 1B.

Models of target delineation for radiosurgery of spine metastasis. (A) Involvement of vertebral body. (B) Extensive vertebral body and pedicle involvement. (C) Involvement of dorsal elements. Adapted from Ryu et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer 2007;109:628-36 [10].
3. Delineation of the spinal cord and normal tissues
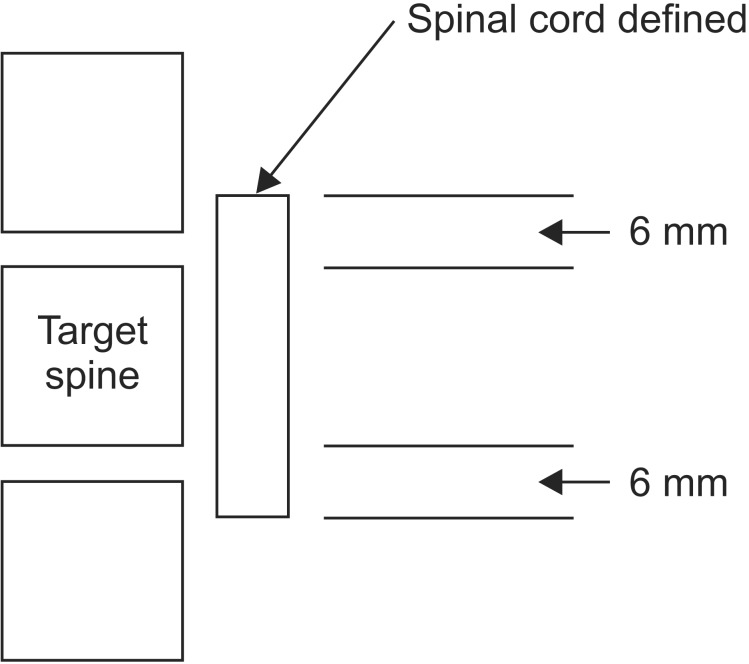
The most critical normal structure for spine radiosurgery is the spinal cord. Planning simulation CT images using 1-3 mm slice thickness are used for delineation of the spinal cord with MR image fusion. T1-weighted contrast-enhanced and T2-weighted images are useful. At the author's institution, the spinal cord volume has been consistently defined as the volume extending from 6 mm from above the target volume to 6 mm below the radiosurgery target. This method of spinal cord volume delineation is diagrammatically shown in Fig. 2. The rationale for this denominator spinal cord volume is based on the distance of dose fall-off (90% to 50% isodose line) being 5 mm, and that the radiosurgical beam arrangement is co-planar [7]. Using this spinal cord volume as denominator, the spinal cord partial volume tolerance dose has been defined as 10 Gy to the 10% of the spinal cord volume defined from 6 mm above to 6 mm below the radiosurgery target volume [3]. When the cord volume receiving greater than 10 Gy was calculated, it was found to be 0.35 mL. A careful precaution is advised in applying the absolute volume tolerance dose because there can be a broad individual variation in spinal cord diameter across patients.
It is also recommended to delineate the normal tissues near the target. These include mucosal structures of laryngopharynx, trachea, and esophagus, as well as bowel and kidney. Recommended normal tissue doses have been also published [11,12].
Evaluation and Patient Selection for Spine Radiosurgery
1. Oncological evaluation of spine metastasis
There has been virtually no treatment algorithm in the evaluation and management of spine metastasis and/or cord compression because it was only approached with the simple thought of palliation. A few treatment algorithms [13,14] focused only on the surgical treatment, and failed to take into account recent advances in treatment thus rendering them less useful. A recent interdisciplinary decision-making framework has been proposed with an acronym of NOMS, which incorporated components of neurologic, oncologic, mechanical instability, and systemic disease [15,16]. The neurologic consideration assesses the presence of myelopathy and functional radiculopathy, but the most critical determinant is the degree of epidural spinal cord compression. The oncologic assessment is predicated on the expected tumor response and durable control to any available treatments as well as consideration of the natural behavior of primary histology. Mechanical instability is a critical decision point as no amount of radiation or chemotherapy will stabilize an unstable spine. A scale of spinal instability secondary to pathologic fractures, called Spinal Instability Neoplastic Score (SINS) has been proposed examining the radiographs and clinical histories with location of neoplasm, characterization of pain, type of bone lesion, radiographic spinal alignment, degree of vertebral body collapse, and involvement of posterolateral spinal elements [17]. Once instability is determined, the initial treatment is typically an interventional procedure such as, open surgical stabilization, percutaneous cement augmentation and/or pedicle screws. However, this also has to be determined based on the extent of systemic disease and medical comorbidities that impact the ability to deliver the proposed treatment and the expected survival.
2. How many spine levels can be treated with spine radiosurgery?
The primary goal of spine radiosurgery is to maximize local tumor control of the involved spine while preserving neurologic function. Like the brain radiosurgery, solitary spine metastasis ('a') is the obvious indication as shown in Fig. 3. Any soft tissue extension of the tumor causing epidural compression or a paraspinal mass is included in the target volume. Two contiguous spine levels can also be treated with radiosurgery ('b' in Fig. 3). In the same token, radiosurgery can be used to treat separate isolated spine metastases that are not adjacent to one another ('c' in Fig. 3). With more common use of MRI for making diagnosis of spine metastasis, there often are multiple micrometastases within the spinal column. These patients are also eligible for radiosurgery when there are limited (1-3) numbers of gross involvement ('d' in Fig. 3). These 4 clinical conditions are included in the ongoing randomized RTOG 0631 trial. In the author's institution, radiosurgery is also utilized for symptom control (i.e., palliative purpose) in patients with diffuse spine metastasis if the symptomatic site was identifiable radiographically and clinically at one or two spine segments. There may be no limited number of spine metastases that can be treated with radiosurgery. It requires caution since the spinal cord dose tends to be higher when the length of target was >6 cm [2], although it can be minimized with the use of radiation intensity modulation.
Treatment algorithm of radiosurgery for spine metastasis. a, Solitary spine metastasis with or without spinal cord compression (red); b, two contiguous spine involvement; c, detached metastases; d, limited number of spinal metastases with scattered small metastases detected on MRI. Adapted from RTOG 0631 trial.
3. Grading system of spinal cord compression
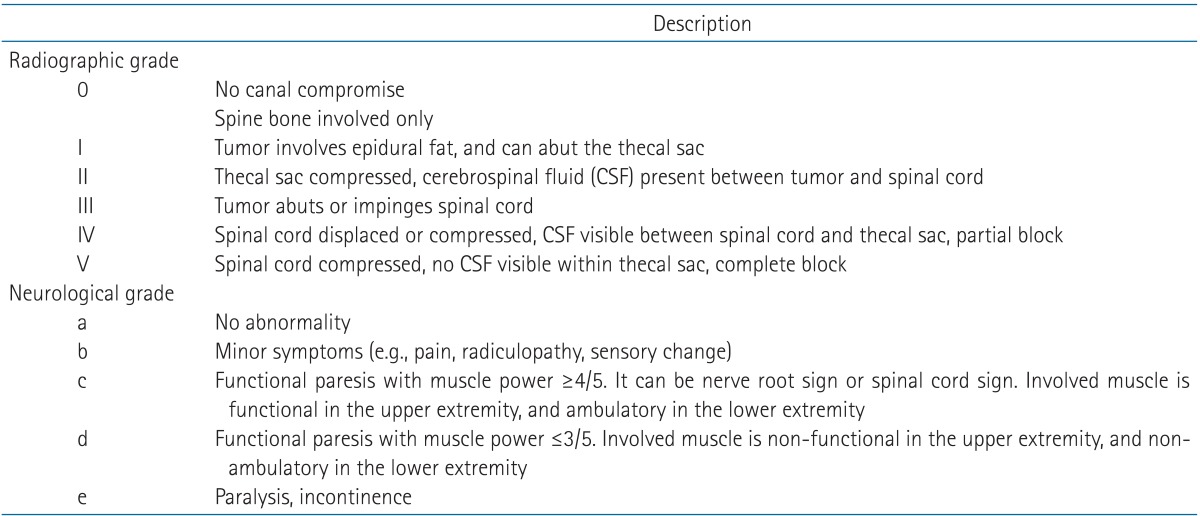
In the process of making a treatment decision, it is important to view the natural process as a spectrum ranging from vertebral bone involvement to mild or moderate epidural compression to frank spinal cord compression. One has to also consider rapidity and severity of neurological symptom development, oncological condition, medical and general condition including comorbidity as well as unique spine problems, such as degenerative change and instability particularly in the elderly spines. Recently, MRI has been used to visualize the extent of such involvement. Treatment decision to treat with surgery is commonly made based on the imaging study rather than neurological status. However, imaging studies are not predictive of the patient's neurological condition. With the use of modern radiosurgery, there are currently two grading systems proposed; one is proposed by Bilsky et al. [18] and it is based on MR imaging study, and the other is based by Ryu et al. [19] and it is based on both MR imaging and neurological status. While no grading system is perfect, the proposed systems provide physicians with a common language across disciplines. Since any imaging study alone is not predictive of neurological status, dual grading system is preferred for evaluation of spinal cord compression. The dual radiographic (anatomical) and neurological (functional) grading system can be used to directly assess the patient and select radiosurgical versus neurosurgical candidates. These are summarized in Table 1, and diagrammatically illustrated in Fig. 4 with examples of images.

Diagram and MRI examples of radiographic epidural compression and examples (irregular gray, tumor; round blue line, thecal sac; round green, spinal cord). Adapted from Gerszten PC, Ryu S. Spine radiosurgery (2nd ed.). New York: Thieme; 2015 in print.
The radiographic grade is based on the imaging studies, based on T1-weighted contrast MR image for epidural tumor and T2-weighted image for assessment of the thecal sac and the spinal cord. The radiographic grade of the lesion ranges from 0 to V. Grade 0 is vertebral bone involvement only without canal compromise. Grade I represents minimal involvement of the epidural fat but without thecal sac displacement. Grade II is impingement and/or displacement of the thecal sac. Grade III represents impingement of the spinal cord but without significant distortion or displacement of the spinal cord. Grade IV represents partial spinal cord compression and displacement with cerebrospinal fluid (CSF) still visible within the thecal sac (so-called partial block on the myelogram). Grade V represents severe spinal cord compression with CSF not visible at the level of compression (complete block). At the level of the cauda, an epidural lesion is deemed grade II when less than 50% of the spinal canal is compressed, and grade IV when more than 50% of the canal is compressed.
The neurological grade is based on the neurological symptoms and the status of the patient at the time of presentation. The neurological grade also consists of five scales (from a to e) based on the neurological status: a , no symptoms; b, a focal minor symptom (i.e., axial pain or radiculopathy); c, functional paresis (i.e., the involved neuromuscular elements compromised but remain partially functional) with muscle strength of ≥4 out of 5 either due to compression of nerve roots or spinal cord; d, nonfunctional paresis (i.e., the involved neuromuscular elements are nonfunctional) with muscle strength ≤ 3 out of 5 either due to compression of nerve roots or spinal cord; and e, complete paralysis or urinary and rectal incontinence.
4. Treatment decision-making
Treatment options for spinal cord compression remain the same. Steroids are used for acute improvement and prevention of neurological symptoms. cEBRT is also used for most of the widespread spine metastases with or without canal compromise. The role of surgery has been demonstrated. Surgery is to achieve decompression of rapidly developing spinal cord compression, and restore the neurological function. The goal of spine radiosurgery is to preserve and improve the neurological status. Therefore, it is reasonable and logical to consider radiosurgery when the patient's neurological condition is well preserved. Ironically, this is quite opposite of what is actually being done in clinical practice at this time, i.e., surgery is used for neurologically intact patient. Comparative neurological outcome results of surgery versus radiosurgery are described in the Section 5.2. Radiosensitivity of the tumor did not make any difference. It is clear that the surgery is indicated when there is neurological deficit, but radiosurgery can be the choice of treatment for any patients who are neurologically intact or ambulatory or have a minor deficit, i.e., for patients with neurological grade c or better.
Treatment Outcome of Radiosurgery
1. Pain control
The usual presenting symptom of spine metastases is back pain. Therefore, the purpose of spine radiosurgery has been primarily for pain control. Pain control of spine metastasis by radiosurgery has initially been reported in the range of approximately 90% using different radiosurgery methods [20,21]. The optimum dose of radiosurgery necessary to achieve pain control is not clearly defined at this time. In the analysis of an early phase II trial of dose escalation at Henry Ford Hospital with dose escalation, there was a strong trend towards increased pain control with a radiation dose of higher than 14 Gy. The one year actuarial pain control rate was 84% with doses higher than 14 Gy delivered in a single fraction [21]. Successful pain control has been reported consistently higher than 90% with the use of various radiosurgery dose in the range of 16.24 Gy regardless of the tumor histology [22,23,24]. This is considerably higher than the average pain control rate of 50%.60% by single dose conventional radiation of 8.10 Gy [25,26]. The current RTOG trial directly compares the conventional 8 Gy (3-dimensional treatment allowed) versus radiosurgery 16 or 18 Gy.
Durability of pain control after radiosurgery is another important parameter. In patients with solitary spine metastasis treated with radiosurgery, the actuarial median duration of pain control was 13 months [21]. The durable pain control after radiosurgery is also superior to pain control after lower dose radiation with 8 Gy where majority of patients experience recurrent pain after 3 months [26], as was demonstrated in RTOG 9714.
2. Control of epidural spinal cord compression and neurological outcome
1) Definitive radiosurgery for spinal cord compression
The goal of treatment for spinal cord compression is epidural tumor control, decompression of the spinal cord, and neurological improvement or preservation. Ryu et al. [19] demonstrated excellent spinal cord decompression and neurological improvement in a phase II trial. This clinical trial examined the use of radiosurgery as a single modality for spinal cord compression, and enrolled patients with high-grade spinal cord compression and motor strength 4/5 or higher. The target volume encompassed the involved spine and epidural or paraspinal tumor component. The radiation dose ranged from 16.20 Gy in a single fraction. There was an approximately 70% reduction of volume in epidural tumors. Overall epidural tumor volume decreased by 65% at 2 months with complete tumor disappearance of 35%. The overall rate of neurologic improvement or preservation was 84% after radiosurgery. The results indicate that the epidural space can be decompressed using radiosurgery.
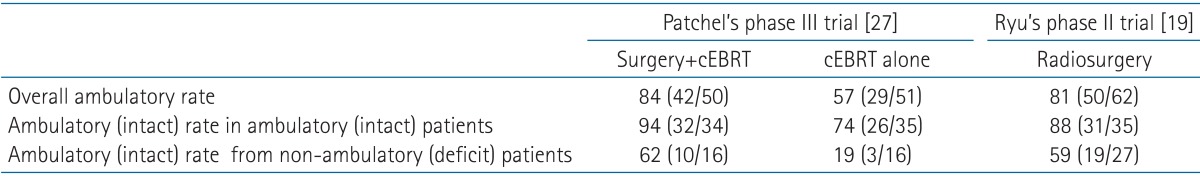
There has been no clinical trial comparing the role of surgery versus radiosurgery for spinal cord compression. In terms of defining the role of surgery, Patchell et al. [27], demonstrated the role of surgical resection plus cEBRT compared with cEBRT alone. Ryu's trial demonstrated the definitive role of radiosurgery [19]. Note that both trials enrolled almost the same patient population; ambulatory patients in Patchell's trial and neurological intact patients in Ryu's trial (more specifically neurological grade c or better). Looking at the arm of surgery plus EBRT (Patchell's trial) and the radiosurgery alone (Ryu's trial), the overall ambulatory rate was 81%.84% in both studies. Subsets of ambulatory (neurologically intact) patients remaining ambulatory (intact) was 88%.94%, whereas non-ambulatory (deficit) patients improving to ambulatory (intact) status was 59%.62%, in both studies. These are summarized in Table 2. Although one cannot directly compare two different studies, the results shed new light on the effect of radiosurgery and selection of treatments. As experience with radiosurgery for spinal cord compression grows, one emerging new question is whether radiosurgery can be used for higher radiographic grades IV.V. Lee et al. [28] analyzed the neurological outcome of radiosurgery for high-grade spinal cord compression. The intact neurological outcome was 80% and radiographic decompression was achieved in 70%. Of note, neurological status was the only prognostic factor for treatment outcome.
The next question is when direct decompressive surgery (no simple laminectomy) or upfront radiosurgery should be used, and what the selection criteria should be. Surgical resection can provide immediate spinal cord decompression, whereas the effect of radiosurgical decompression takes place gradually. Therefore, an argument could be made that patients with minimal neurological signs (i.e., ambulatory patients) can be treated with radiosurgery, and surgery can be reserved for those who progress. It seems logical to use radiosurgery in patients with intact status (grades a, b) and with minimal deficit (grade c), and surgery in patients with overt deficits (grades d, e) and with rapid progression of symptoms. In this regard, the dual grading system is useful and provides good guidance. Other factors to consider are the patient's general oncological condition and tolerability of open surgery or radiosurgery.
The strategy of salvage surgery for tumor progression after radiosurgery may raise a question about potential increase in postoperative complication and delay in wound healing. This may not be of concern because radiosurgery is given only to the involved spine and the nature of radiosurgery where radiation dose accumulation is immediately around the target. Therefore, most of the surgical field is likely larger and outside of the radiosurgery volume. In addition, the usual palliative surgery in the form of laminectomy is also outside of radiosurgery bed.
2) Postoperative radiosurgery or separation surgery
Postoperative radiosurgery has been also used. It is clear that surgery alone is not sufficient for durable tumor control, and that adjuvant radiation treatment is always required [27]. Rock et al. [29] initially reported the first experience with postoperative radiosurgery following open surgery. Radiosurgery was performed usually one to two weeks after open surgery. The radiosurgery target volume included the residual spine tumor and to the surgical bed. One drawback of postoperative radiosurgery is difficulty in delineating the tumor and the spinal cord due to poor image quality secondary to the interference of hardware used for spine stabilization. Despite this, 92% local tumor control rate was reported and patients remained neurologically stable or improved. The procedure was well tolerated and associated with little or no significant morbidity.
There has been concern of under-dosing a very small portion of the epidural lesion immediately adjacent to the spinal cord. In order to permit optimal radiosurgical dosing to the tumor, a small (2 mm) separation between tumor and spinal cord can be helpful. The idea of 'separation surgery' was devised, in which only minimal tumor resection is carried out to separate the tumor margin from the spinal cord, leaving the bulk of the tumor mass to be treated with radiosurgery. Surgery only needs to provide separation between the tumor and the spinal cord supported by a posterior instrumented fusion to optimize the delivery of radiosurgery. It appears that local tumor progression is in the range of 4%-9% after instrumented separation surgery followed by various regimens of single 24 Gy or fractionated (8-10 Gy × 3) radiosurgery in radioresistant tumors [30,31]. This strategy may be useful in radioresistant tumors which may require high radiation dose for tumor control.
Treatment Failure and Complications
In order to maximize likelihood of successful clinical response to spine radiosurgery while minimizing toxicity profile, it is helpful to carefully analyze patterns of treatment failure and complications of treatment. Because of the steep dose gradient, treatment failure must be carefully correlated to the dosimetric profile of each case. With the accumulated experience in spine radiosurgery, it is reassuring to know that the complication rate for spine radiosurgery is relatively low.
1. Patterns of failure after radiosurgery
Treatment failures after spine radiosurgery can be divided into three different categories. In-field failure refers to tumor regrowth inside the target volume. This may be related to the radiosurgical dose necessary for tumor control or inherent radiosensitivity of the tumor. Marginal failure is a failure within the region of rapid dose fall-off immediately outside of the target volume. Causes of marginal failure could be geographical miss or patient set up error, or underestimation of target volume. Distant failure is due to progressive metastatic spread involving the untreated vertebra in the spinal column.
Results of failure after radiosurgery showed an infield failure rate of 6%. More importantly, there was only a 5% incidence of failure at the immediately adjacent spine (marginal failure) [32,33]. The low incidence of infield or marginal failure justifies the use of radiosurgery for localized spine metastasis. Though persistent or progressive pain may appear to be attributed to tumor progression at the treated spine, other causes such as spine instability and degenerative disorder should also be ruled out. It is advisable to minimize the likelihood of developing symptoms secondary to structural problems in the spinal column.
2. Neurological complications
Acute worsening of pain can occur in about 20% range. This pain flare may occur within 24 hours of single dose of radiosurgery, or after a few days with fractionated regimen [34,35]. It is usually transient. It is seen as a local sharp pain or radicular in nature. Short-term use of glucocorticoid can control this pain flare. Prophylactic use of steroid is not indicated. The cause of pain flare is not understood. It may be due to acute radiation-induced inflammation of the nerve roots. It is advised to define the nerve roots and minimize the radiation dose in these areas.
The potential consequences of radiation-induced spinal cord injury can be severe. The best way to prevent radiation-induced myelopathy is to avoid unnecessary or excessive radiation to the spinal cord. The tolerance dose of the spinal cord using traditional external beam radiation is 45.50 Gy in 25 fractions given across the entire diameter of the spinal cord. Biologically effective dose (BED) calculation has commonly been performed to estimate the radiosurgical tolerance dose. However, this is not applicable to spine radiosurgery because there is a sharp dose fall-off within the spinal cord.
In a large clinical trial of 230 patients with careful documentation of spinal cord dose, the spinal cord partial volume tolerance dose has been defined as 10 Gy to the 10% partial volume of the spinal cord defined from 6 mm above to 6 mm below the radiosurgery target volume [2]. When the absolute volume receiving higher than 10 Gy was calculated, it was found to be 0.35 mL. A careful precaution is advised in applying the absolute volume tolerance dose because there is potentially a large individual variation in spinal cord diameter across patients. See Section 3.3 above. The ongoing RTOG 0631 randomized trial adopted this dose constraint. There was one incidence of spinal cord damage clinically and radiographically 13 months after 16 Gy radiosurgery for C1 and C2 epidural compression from the primary breast cancer. The patient improved with steroid treatment. Since spinal cord complications can occur after a long latency period, long-term follow-up is certainly needed.
3. Non-neurological complications
Vertebral compression fracture has been reported with approximately 20% incidence. It is more often seen in lytic metastases on imaging studies involving more than half of the vertebral body, with radiosurgical dose >20 Gy [36,37]. For compression fractures, one has to also consider whether it is caused by oncological condition or other degenerative changes particularly in elderly spines, and whether it is symptomatic or not. It is important to remember that compression fracture is not a contraindication of spine radiosurgery. Instead, it is important to consider the entire clinical picture, which includes spine stability and general oncological condition.
Normal tissues should be delineated depending on the level of the involved vertebral body, such as the larynx, pharynx, esophagus, bowel, lung, or kidney, etc. The tolerance dose of these organs to radiosurgery is not defined. Experience of certain organ tolerances have been reported, such as for mucositis of the pharynx or esophagus in cervical or upper thoracic spine treatments [11,12]. These symptoms manifest as odynophagia or dysphagia. Acute side effects associated with mucositis generally resolve within two weeks. Long-term tracheoesophageal fistula has also been reported in an immunocompromized patient with multiple myeloma after multiple chemotherapy regimens and bone marrow transplantation. Skin reactions are seldom seen after radiosurgery unless the tumor involves the posterior element extending close to skin. Every effort should be given to minimize any unnecessary radiation dose to the adjacent tissues.
There are many factors that may influence radiosurgical complications. These may include proximity and the extension of the tumor to the adjacent normal tissues, concurrent systemic therapy, compounding comorbidities such as acute infection, prior surgery, and host factors such as diabetes, collagen vascular disease, or any genetic predisposition to the radiosensitivity. A careful assessment of the patient's clinical condition and dosimetry of radiosurgery are necessary in order to reduce the potential risk of radiation-induced complications.
Combined Modality Approach
The combined use of radiosurgery with open surgery or separation surgery has been described above. Vertebroplasty or kyphoplasty can be used together or in sequence. The experience of the combined procedure at the University of Pittsburgh was well tolerated with excellent results of pain control. Radiosurgery was usually given within one to two weeks after vertebroplasty [38]. Vertebroplasty or kyphoplasty is considered when there is concern regarding spine instability or compression fracture that might be causing pain. However, it is important to realize they are not oncological procedures. Therefore, it is recommended to perform radiosurgery first for oncological management, prior to the surgical procedure. It is important not to use kyphoplasty for any type of spinal cord compression as it can actually exacerbate retropulsion of the epidural tumor.
Combined treatment with chemotherapy or emerging biological therapies has not been well explored. Since radiosurgery is usually given in a single or few fractions to the involved spine, concurrent chemotherapy schedules are not usually altered. Common practice is to hold chemotherapy on the day of radiosurgery. There is no supporting data for concurrent chemotherapy with radiosurgery at this time. Delayed recall phenomenon by sorafenib has been reported 8 weeks after radiosurgery [39]. A special precaution should be made for potential interaction between drug therapy and radiation. Indeed, a more practical advantage of radiosurgery is that the functioning red marrow can be preserved in patients who need systemic chemotherapy.
Tumors involving the spinal column are complex. They manifest not only with symptoms of pain or neurological deficits but also with other general oncological problems, or even with many socio-economic issues. In addition, any symptoms arising from the weight bearing axial skeleton directly affect the function and the quality of life. Additional complicating issues include intercurrent degenerative changes, osteoporosis and associated bone changes, and spine instability problems. In order to deal with these issues a multidisciplinary approach is important to promote interdisciplinary group discussion, thereby providing comprehensive care for spine tumor patients.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.