Prognostic value of nodal SUVmax of 18F-FDG PET/CT in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy
Article information
Abstract
Purpose
To investigate the predictive role of maximum standardized uptake value (SUVmax) of 2-[18F]fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in nasopharyngeal cancer patients treated with intensity-modulated radiotherapy (IMRT).
Materials and Methods
Between October 2006 and April 2016, 53 patients were treated with IMRT in two institutions and their PET/CT at the time of diagnosis was reviewed. The SUVmax of their nasopharyngeal lesions and metastatic lymph nodes (LN) was recorded. IMRT was delivered using helical tomotherapy. All patients except for one were treated with concurrent chemoradiation therapy (CCRT). Correlations between SUVmax and patients’ survival and recurrence were analyzed.
Results
At a median follow-up time of 31.5 months (range, 3.4 to 98.7 months), the 3-year overall survival (OS) and disease-free survival (DFS) rates were 83.2% and 77.5%, respectively. In univariate analysis, patients with a higher nodal pre-treatment SUVmax (≥ 13.4) demonstrated significantly lower 3-year OS (93.1% vs. 55.5%; p = 0.003), DFS (92.7% vs. 38.5%; p < 0.001), locoregional recurrence-free survival (100% vs. 50.5%; p < 0.001), and distant metastasis-free survival (100% vs. 69.2%; p = 0.004), respectively. In multivariate analysis, high pre-treatment nodal SUVmax (≥ 13.4) was a negative prognostic factor for OS (hazard ratio [HR], 7.799; 95% confidence interval [CI], 1.506–40.397; p = 0.014) and DFS (HR, 9.392; 95% CI, 1.989–44.339; p = 0.005).
conclusions
High pre-treatment nodal SUVmax was an independent prognosticator of survival and disease progression in nasopharyngeal carcinoma patients treated with IMRT in our cohort. Therefore, nodal SUVmax may provide important information for identifying patients who require more aggressive treatment.
Introduction
Overall, survival and local control of nasopharyngeal cancer patients have both significantly improved due to advances in diagnostic imaging and the introduction of systemic chemotherapy and intensity-modulated radiotherapy (IMRT), but distant metastasis is still a major cause of treatment failure [1-3]. Therefore, early identification of patients with a high risk of disease progression before treatment is very important because administration of individualized therapy to these patients may improve their clinical outcome. As current prognostic factors are limited when identifying high-risk patients who require more aggressive treatment [4-6], newer prognostic factors for clarifying the risk stratification of patients are needed.
Maximum standardized uptake value (SUVmax) of 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is an index that reflects tumor metabolism. The clinical significance of a high SUVmax has already been identified for many carcinomas such as breast and lung cancer [7,8]. In addition, the findings of several studies have suggested poorer prognosis in nasopharyngeal cancer patients with a higher SUVmax [9-15]. Accordingly, we conducted a study to clarify the prognostic significance of high pre-treatment SUVmax and evaluated the significance of the SUVmax in both primary site and metastatic nodes.
Materials and Methods
1. Study patients
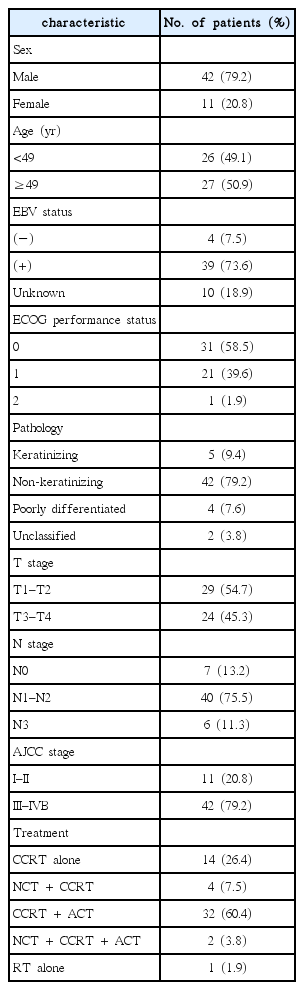
Between October 2006 and April 2016, 67 nasopharyngeal patients were initially treated with IMRT in two institutions. We retrospectively analyzed these patients’ medical records and excluded patients with distant metastasis at the time of diagnosis or who had another malignancy. In addition, patients who had previously received radiotherapy at another hospital and did not complete the planned radiation therapy were excluded. Finally, 53 patients were included in this study that met the following criteria: (1) biopsy-proven nasopharyngeal carcinoma; (2) stage I to IVB according to the American Joint Committee on Cancer (AJCC) staging, 7th edition; and (3) underwent treatment with IMRT using helical tomotherapy (Accuray Inc., Sunnyvale, CA, USA).
All patients underwent a complete medical history taking and physical examination at the time of diagnosis. Fiberoptic nasopharyngoscopy, complete blood count (CBC), and baseline blood chemistry were also performed. Neck CT and/or neck magnetic resonance imaging (MRI), chest radiography with chest CT, abdominal CT, a bone scan, and PET/CT were performed for staging evaluation.
We reviewed the medical records and diagnostic images of all patients, and investigated prognostic factors including SUVmax. All patients were re-staged according to the 7th edition of the AJCC. We reviewed neck MRI or neck CT (if neck MRI was not performed) for evaluating the node size of patients with LN metastasis, and evaluated the longest diameter of the coronal (long axis) and axial scan (short axis) for the largest metastatic nodes. Nodal size was evaluated in 40 patients, excluding 6 patients whose initial imaging was insufficient. We retrospectively reviewed the initial PET/CT of all patients and the pre-treatment SUVmax of the primary nasopharyngeal lesion and metastatic nodes. The pre-treatment SUVmax of the primary site was identified for all 53 patients. Because 7 patients did not have any metastatic lymph nodes (N0) or did not have a numerical value of the SUVmax of metastatic LNs recorded, pre-treatment nodal SUVmax was identified for 46 patients. For patients with multiple metastatic nodes, the highest SUVmax value among several nodal lesions was selected as the nodal SUVmax. We also reviewed PET/CT performed within 3 months after completion of RT, and we examined the post-RT metabolic response in 26 patients.
2. Protocol of PET/CT and imaging analysis
All patients underwent PET/CT before treatment. Both institutions used the same PET/CT protocol (fasting duration, pre-injected blood glucose level, amount of injected 18F-FDG, post-injection interval), but the PET/CT scanner used was different—one institution used Biograph Duo (Siemens Medical Solutions, Knoxville, TN, USA) and the other institution used Discovery STE (GE Healthcare Inc., Milwaukee, WI, USA). Subjects fasted for at least 6 hours before PET/CT scans, and their blood glucose levels were measured before injection of 18F-FDG. None of the patients had blood glucose levels greater than 130 mg/dL before injection. A dose of 5.5–7.4 MBq/kg of FDG was administered intravenously and scanning began 60 minutes after injection. The CT scan began at the orbitomeatal line and progressed to the proximal thigh (Biograph Duo: 130 kVp, 80 mA, and 5 mm slice thickness; Discovery STE: 140 kVp, auto mA, and 3.75 mm slice thickness). The PET scan followed immediately over the same body region. The CT data were used for attenuation correction, and images were reconstructed using a standard ordered-subset expectation maximization (OSEM) algorithm (2 iterations, 8 subsets). The axial resolution was 6.5 or 4.5 mm at the center of the field of view.
All PET/CT images were analyzed by experienced nuclear medicine physicians. The metabolic activity of any lesion with a visually abnormal FDG uptake was analyzed using the standardized uptake value (SUV). SUV was calculated by the following formula:
SUVmax of 18F-FDG was measured by visually placing the region of interest (ROI) around the site of increased FDG uptake.
3. Radiotherapy
For simulation and treatment, patients were placed in the supine position and immobilized from head to neck with a thermoplastic mask (CIVCO Radiotherapy Inc., Coralville, IA, USA). A CT simulation was performed with a slice thickness of 2.5 mm extending from the vertex to the upper chest using a LightSpeed RT 16 CT scanner (GE Healthcare Inc.). All patients were treated with IMRT with a radical aim with a 6-MV photon beam using helical tomotherapy, 5 days per week. Gross tumor volume (GTV) was delineated based on enhanced neck CT, neck MRI, and PET/CT. IMRT was performed by using a simultaneous integrated boost technique for each GTV of the nasopharynx and metastatic nodes, and clinical target volume (CTV) 1, 2, and 3. CTV1 was defined as the GTV of the nasopharynx plus a 5-mm to 1-cm margin to cover the risky sites of microscopic tumor cell infiltration around the GTV and anatomic extension of the nasopharynx. CTV2 was defined as the high-risk region of lymph node metastasis (both II, III, and Va), and CTV3 was defined as the low-risk region of lymph node metastasis (both IV and Vb). The planning target volume (PTV) was defined as the 3–5 mm margin of each CTV. The prescription dosage was 68–76 Gy/32–36 fractions (fraction size, 2.12–2.3 Gy) to the GTV of the primary site and metastatic nodes, respectively, 60–66 Gy/32–36 fractions (fraction size, 1.8–2 Gy) to CTV1, 57–61 Gy/32–36 fractions (fraction size, 1.7–1.9 Gy) to CTV2, and 50–56 Gy/24–36 fractions (fraction size, 1.6–2.12 Gy) to CTV3. For CTV3, some patients were scheduled for 50.8 Gy/24 fractions (fraction size, 2.12 Gy) or 50 Gy/25 fractions (fraction size, 2.0 Gy) and were excluded from the target volume in a cone down plan. The prescription dose of PTV was 80%–100% of the dose to each CTV. The prescribed dose encompassed at least 95% of the target volume. Critical adjacent structures such as the brainstem, optic nerve, optic chiasm, parotid gland, submandibular gland, and mandible were spared as much as possible so as not to exceed the tolerance dose.
4. Chemotherapy
All patients except for one (who was treated with definitive radiotherapy alone) were treated with concurrent chemoradiation therapy (CCRT). Among these, 14 patients were treated with CCRT alone, 4 patients underwent neoadjuvant chemotherapy, 32 patients underwent adjuvant chemotherapy, and 2 patients underwent both neoadjuvant and adjuvant chemotherapy. Forty-eight out of 52 patients were treated with a cisplatin-based regimen (cisplatin 30 mg/m2 on day 1 and every week for 6 or 7 cycles or cisplatin 100 mg/m2 on day 1 and then every 3 weeks for 3 or 4 cycles during radiotherapy). The other four patients were administered either cisplatin (100 mg/m2)/5-fluorouracil (5-FU; 1,000 mg/m2), carboplatin (30 mg/m2), etoposide (120 mg/m2)/cisplatin (60 mg/m2), or cetuximab, respectively. Cisplatin (100 mg/m2 on day 1)/5-FU (1,000 mg/m2 on days 1–5) was administered every month for 3 or 4 cycles, and docetaxel (70 mg/m2 on day 1)/cisplatin (70 mg/m2 on day 1)/5-FU (700 mg/m2 on days 1–4) were administered every 3 weeks for 2 or 3 cycles, as induction or adjuvant chemotherapy.
5. Patient assessments and follow-up
All patients were evaluated weekly during RT, and then they underwent follow-up every 2–3 months after completion of RT for the first 2 years and every 4–6 months after that. Fiberoptic nasopharyngoscopy, CBC, blood chemistry, and physical examination including neck node palpation were performed at each follow-up. Neck CT or neck MRI was performed one month after the end of RT and PET/CT was performed three months for initial therapeutic response evaluation. Initial therapeutic response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1.
6. Study endpoints and statistical analysis
The primary endpoint of this study was the prognostic value of the SUVmax of PET/CT for treatment outcomes. Secondary end points were overall survival (OS), disease-free survival (DFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS). We analyzed survival, recurrence, and prognostic factors including SUVmax of PET/CT. All statistical analysis was performed using SPSS ver. 18 for Windows, (SPSS Inc., Chicago, IL, USA). Actuarial 3-year OS, DFS, LRRFS, and DMFS were analyzed by the Kaplan-Meier method and the correlation of the survival rates with prognostic factors was analyzed by the log-lank test. Multivariate Cox proportional hazard models for OS and DFS were built with prognostic factors with a p-value of <0.1 in univariate analysis. A p-value less than 0.05 was defined as statistically significant. Chi-square tests and independent-sample t-tests were used to compare characteristics between the two groups classified by the cut-off of 13.4 for SUVmax-n. The correlation between primary site SUVmax and nodal SUVmax, and the correlation between nodal size and nodal SUVmax were analyzed by linear regression. OS, DFS, LRRFS, and DMFS were calculated from the first date of treatment to the date of an event or the last follow-up visit. The endpoint of OS was defined as the occurrence of any death or last follow-up visit and the endpoint of DFS was defined as the occurrence of disease-related death or the diagnosis of a recurrence.
Results
1. Patient characteristics and clinical outcomes
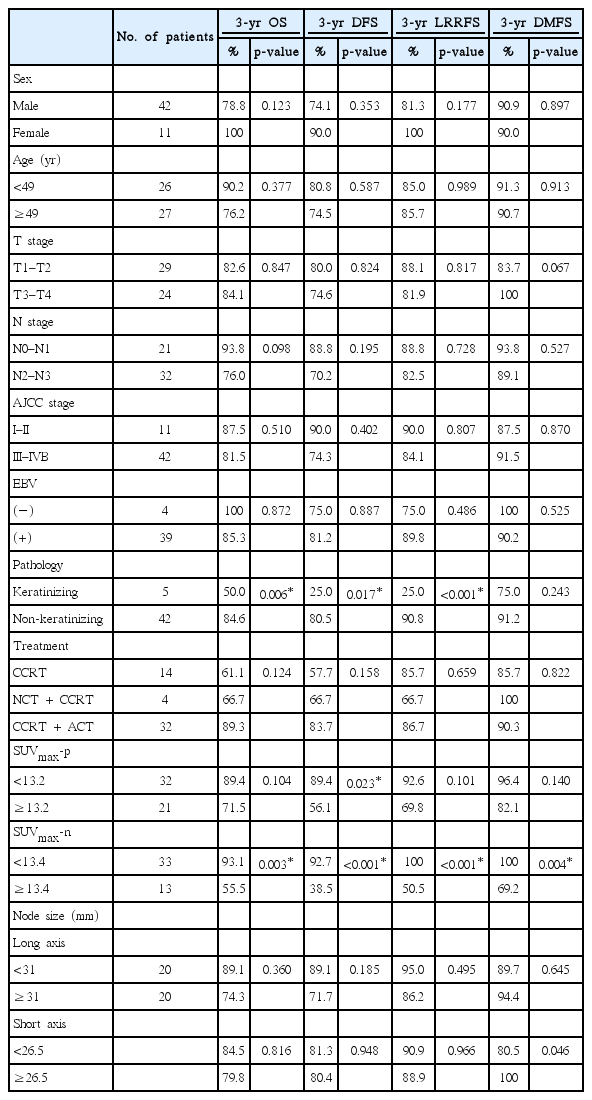
A total of 53 patients who underwent IMRT between October 2006 and April 2016 were analyzed. The median age of this group was 49 years (range, 14 to 75 years) and the majority was men (79.2%). Thirty-nine patients (73.6%) out of 53 patients were positive for Epstein-Barr virus (EBV). Their pathologic classification was based on the 2005 World Health Organization classification. However, when the tissue type was described only as poorly differentiated nasopharyngeal carcinoma, it was categorized as ‘poorly differentiated carcinoma,’ and when the tissue type was described only as a nasopharyngeal carcinoma; it was categorized as ‘unclassified’. The most common pathologic type was non–keratinizing carcinoma (42 patients, 79.2%). Most of the patients (79.2%) had an advanced stage (stage III–IVB) and all patients had a performance status below Eastern Cooperative Oncology Group performance status 2. The patient characteristics are summarized in Table 1. Comparative assessment of the patients’ characteristics between the two groups based on the nodal SUVmax cut-off value of 13.4 did not demonstrate any significant differences (Table 2). The median nodal size of the largest metastatic LN for the long axis was 31 mm (range, 12 to 59.04 mm) and 26 mm (range, 14 to 48.7 mm) for the short axis in the total cohort.
The median follow-up duration was 31.5 months (range, 3.4 to 98.7 months). Eight patients died and 8 patients experienced a recurrence during the follow-up period. Among the patients who experienced a recurrence, there was 1 patient with an isolated local recurrence, 2 patients with a distant recurrence without locoregional recurrence, 3 patients with local and regional recurrences, 1 patient with regional and distant recurrence, and 1 patient with local, regional, and distant recurrences. Recurrence or progression in the lymph node was categorized as a regional recurrence regardless of the presence of lymph node metastasis at initial diagnosis.
Three-year OS, DFS, LRRFS, and DMFS rates were 83.2%, 77.5%, 85.4%, and 90.9%, respectively. There were 28 patients (52.8%) with a complete response, 21 (39.6%) with a partial response, 2 with stable disease, and no patients with progressive disease at initial therapeutic response evaluation. The post-RT metabolic response was evaluated in 26 patients who underwent PET/CT within 3 months after completion of RT. Twelve patients had a metabolic complete response (SUVmax ≤ 2.5), and 14 patients had a metabolic partial response (SUVmax > 2.5); no patients had metabolic stable disease or progressive disease.
2. SUVmax value of primary site and metastatic node and interaction
The pre-treatment SUVmax of the primary site and metastatic lymph node were investigated and were named ‘SUVmax-p’ and ‘SUVmax-n’, respectively. The median SUVmax-p was 11.4 (range, 4.1 to 23.4) and the median SUVmax-n was 9.8 (range, 2.3 to 24.5) in our cohort. The best cut-off value of the SUVmax-p and SUVmax-n was 13.2 (area under the curve [AUC], 0.812; p = 0.010) and 13.4 (AUC, 0.976; p < 0.001) by receiver operating characteristic (ROC) curve analysis for recurrence (Fig. 1). When we analyzed the association between SUVmax-p and SUVmax-n by linear regression, a weak correlation was found between them (R2 = 0.144, p = 0.010, data not shown). In addition, the node size of the short axis and SUVmax-n showed no significant correlation (R2 = 0.118, p = 0.103), while the node size of the long axis and SUVmax-n showed a weak correlation (R2 = 0.106, p = 0.043).

Receiver operating characterstic (ROC) curve of SUVmax of the primary site and metastatic node for predicting recurrence. The best cut-off values for SUVmax-p and SUVmax-n depicted by the ROC curve were 13.2 and 13.4 for recurrence. SUVmax, maximum standardized uptake value; SUVmax-p, pretreatment SUVmax of primary site; SUVmax-n, pretreatment SUVmax of metastatic nodes.
3. Prognostic significance of SUVmax on survival and recurrence
In univariate analysis, pathology, high SUVmax-p (≥13.2), and high SUVmax-n (≥13.4) were significant prognostic factors (Table 3). The non-keratinizing (differentiated and undifferentiated) nasopharyngeal carcinoma pathology group had a significantly higher OS (p = 0.006), DFS (p = 0.017), and LRRFS (p < 0.001) at 3 years than the keratinizing squamous cell carcinoma pathology group. The higher SUVmax-p (≥13.2) group had a significantly lower DFS (p = 0.023) than the lower SUVmax-p group (<13.2). A higher SUVmax-n (≥13.4) was a negative prognostic factor for 3-year OS (93.1% vs. 55.5%; p = 0.003) as well as for DFS (92.7% vs. 38.5%; p < 0.001), LRRFS (100% vs. 50.5%; p < 0.001), and DMFS (100% vs. 69.2%; p = 0.004) (Fig. 2). There was no statistically significant difference between the two groups when classified by median value of largest nodal size. Additionally, a metabolic complete response response did not demonstrate significant results for survival and recurrence in univariate analysis. In multivariate analysis, only a high SUVmax-n (≥13.4) was statistically significant. A high SUVmax-n (≥13.4) was a negative and independent prognostic factor for OS (hazard ratio [HR], 7.799; 95% confidence interval [CI], 1.506–40.397; p = 0.014) as well as for DFS (HR, 9.392; 95% CI, 1.989–44.339; p = 0.005) (Table 4).
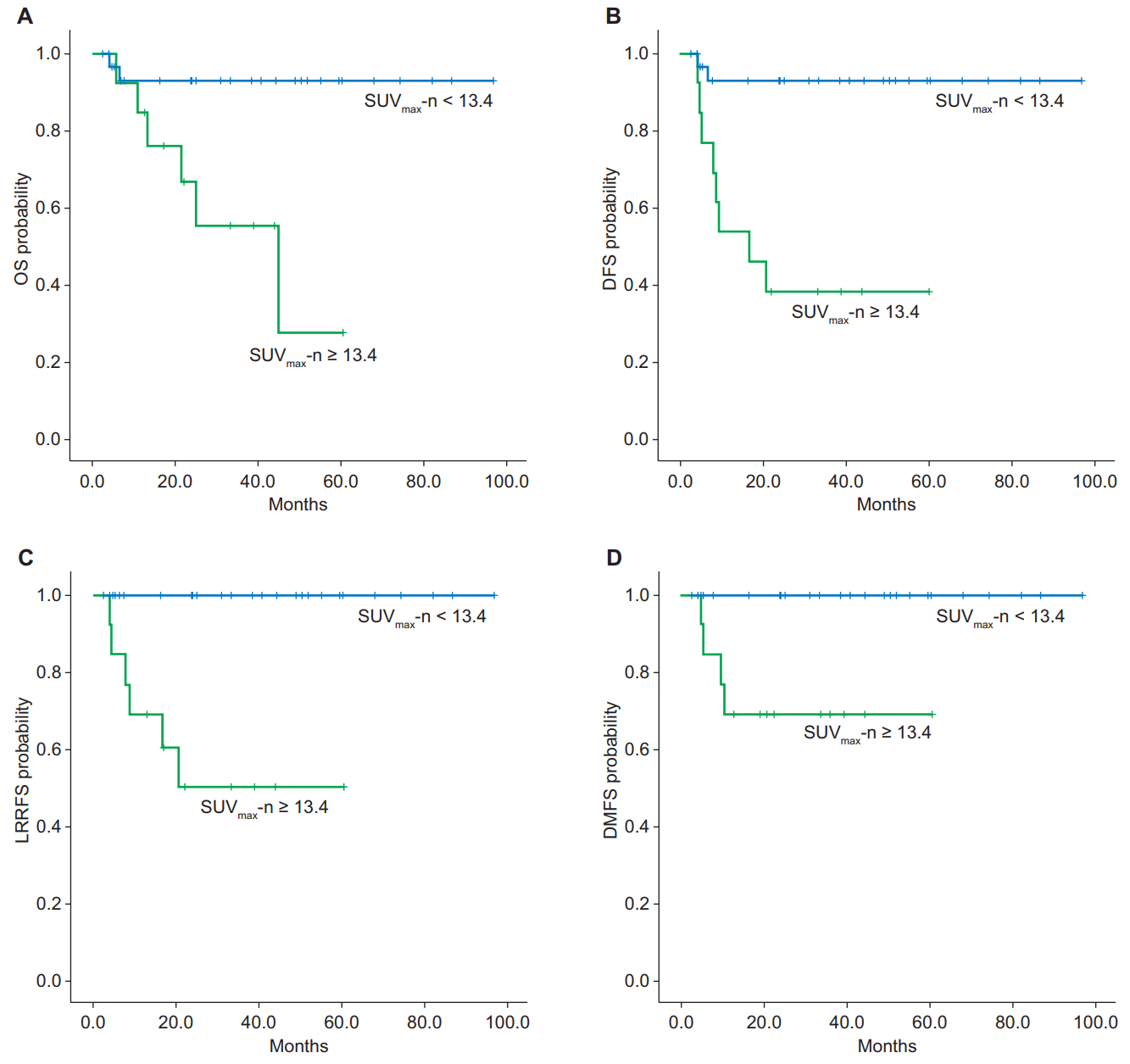
Kaplan-Meier estimates of survival curves for the SUVmax-n < 13.4 and SUVmax-n ≥ 13.4 groups. (A) Overall survival (OS) of two groups (93.1% vs. 55.5%; p = 0.003). (B) Disease-free survival (DFS) of two groups (92.7% vs. 38.5%; p < 0.001). (C) Locoregional recurrence-free survival (LRRFS) of two groups (100% vs. 50.5%; p < 0.001). (D) Distant metastasis-free survival (DMFS) of two groups (100% vs. 69.2%; p = 0.004).
Discussion and Conclusion
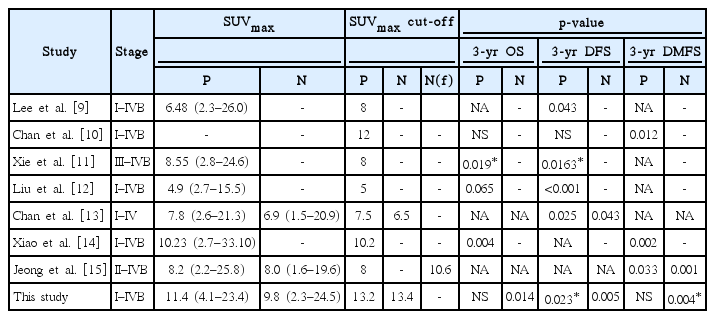
Identifying prognostic factors for individualized therapy in patients with nasopharyngeal carcinoma is currently a major issue, and several studies have been conducted on risk stratification of patients using EBV DNA level and PET/CT parameters [16-20]. Several previous studies have suggested pre treatment SUVmax has prognostic value for nasopharyngeal cancer, as shown in Table 5 [9-15], but for most of these studies, only the SUVmax of the primary site was analyzed. This study focused on the importance of both the nodal and primary site SUVmax, and we found that nodal SUVmax is an independent prognostic factor for OS and DFS. This is consistent with a previous study by Chan et al. [13], in which a higher nodal SUVmax (≥6.5) was a negative prognostic factor for DFS (HR, 4.1; 95% CI, 1.045–16.084; p=0.043) in nasopharyngeal cancer patients. In addition, in a study of 178 patients with head and neck squamous cell carcinoma, that included 28 patients with nasopharyngeal carcinoma, the higher nodal SUVmax (≥6) group had a significantly lower DFS rate (HR, 1.81; 95% CI, 1.02–3.23; p = 0.04) and a DMFS rate (HR, 3.34; 95% CI, 1.25–8.92; p = 0.016). In addition, nodal SUVmax was found to be a stronger prognostic factor than SUVmax of the primary site [21].
In our univariate analysis, SUVmax-n was found to be a significant factor for LRRFS (100% vs. 50.5%; p < 0.001) and DMFS (100% vs. 69.2%; p = 0.004). However, multivariate analysis for SUVmax-n was not performed for LRRFS and DMFS because both locoregional recurrence and distant metastasis only occurred in the higher SUVmax-n group. Therefore, further information about the relationships between SUVmax-n and locoregional recurrence and distant metastasis can be achieved through further study.
Additionally in the univariate analysis, we found the early AJCC stage group (I–II) had a lower DMFS than the advanced stage group (III–IVB). Because early AJCC stage was only observed in one out of 4 patients with distant metastasis, we thought this likely to be a bias caused by the small sample size.
In this study, we did not find any significance of post-RT metabolic response on patients’ survival and recurrence. However, there is a previous study in which a post-treatment metabolic complete response state was found to be a favorable factor for overall survival and DFS [11]. And, because of the limited number of patients, further studies with more patients will be needed.
We did not find an independent association of primary SUVmax with survival and disease progression. When we analyzed the correlation between primary SUVmax and nodal SUVmax, only a weak correlation (R2 = 0.144, p = 0.010) was found. Therefore, nodal SUVmax is a prognostic factor that provides more significant information about the patient’s clinical outcome than the primary SUVmax. In addition, only the long axis nodal size showed a weak correlation with SUVmax-n (R2 = 0.106, p = 0.043), unlike the short axis node size. This suggests that nodal SUVmax is a factor that reflects biologic aggressiveness of nodal metastasis and can predict the prognosis of patients independently of node size.
In this study, we used a relatively high cut-off value (SUVmax-p, 13.2; SUVmax-n, 13.4) compared with previous studies on SUVmax in nasopharyngeal carcinoma. Compared with other studies, the median SUVmax itself was relatively higher, as shown in Table 5 [9-15]. This may be because of the measuring protocol and device used, and may be a reflection of the tumor burden, because 80% of the study objects were in an advanced stage.
This study has several limitations. First, as a retrospective study, there could be a selection bias for treatment strategy and chemotherapy that was performed heterogeneously among patients. Second, it includes a relatively low number of patients and few events. Third, there may have been few inconsistencies in the SUVmax because two different PET scanners were used, although they used the same protocol. In general, SUV measurements may vary from institution to institution depending on the differences in PET/CT protocols, PET/CT scanners, and imaging analysis methods. This imposes limitations on reproducibility. Because of these limitations, the optimal cut-off value of this study may not consistently be the best discrimination value in other patient groups. In addition, SUVmax has a limitation in that it does not reflect the heterogeneity of the total tumor lesion and volume.
To overcome these limitations, studies using prognostic factors such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) of PET/CT have recently been proposed. Yoon et al. [22] reported high MTV (≥31.45 cm3 if set to cut-off 2.5; ≥23.01 cm3 if set to cut-off 3.0) was a negative prognostic factor for OS (HR, 3.7019; 95% CI, 0.4746–9.3602; p = 0.0453 and HR, 5.1274; 95% CI, 1.1594–15.6541; p = 0.0198) in nasopharyngeal cancer patients. The TLG is the value obtained by multiplying the SUVmean by MTV; it reflects the volumetric factor as well as biologic activity of the whole tumor. In a study by Chan et al. [19] high TLG (>330) was found to be a negative prognostic factor for OS (HR, 1.0013; 95% CI, 1.0005–1.0021; p = 0.0014) and DFS (HR, 3.0263; 95% CI, 1.6307–5.6164; p = 0.0005). Theoretically, TLG reflects the disease activity of the entire tumor lesion and its volume factor may be superior to SUVmax or MTV in prognostication, but this has not yet been proven [20,23,24]. In a recent meta-analysis of 18F-FDG-PET/CT in nasopharyngeal carcinoma by Lin et al. [25] SUV, MTV, and TLG were found to be significant prognostic factors for OS and event-free survival, respectively. Although MTV and TLG were not analyzed in this study, the prognostic significance of SUVmax has been demonstrated several times in previous studies [9-15]. The results of this study were meaningful in suggesting the importance of nodal SUVmax, unlike previous studies focusing on primary tumors.
In conclusion, high pre-treatment nodal SUVmax was an independent prognosticator of survival and disease progression in nasopharyngeal carcinoma patients treated with IMRT in our cohort. Therefore, it may provide important information for identifying patients who require more aggressive treatment.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.