Image-guided radiation therapy in lymphoma management
Article information
Abstract
Image-guided radiation therapy (IGRT) is a process of incorporating imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), Positron emission tomography (PET), and ultrasound (US) during radiation therapy (RT) to improve treatment accuracy. It allows real-time or near real-time visualization of anatomical information to ensure that the target is in its position as planned. In addition, changes in tumor volume and location due to organ motion during treatment can be also compensated. IGRT has been gaining popularity and acceptance rapidly in RT over the past 10 years, and many published data have been reported on prostate, bladder, head and neck, and gastrointestinal cancers. However, the role of IGRT in lymphoma management is not well defined as there are only very limited published data currently available. The scope of this paper is to review the current use of IGRT in the management of lymphoma. The technical and clinical aspects of IGRT, lymphoma imaging studies, the current role of IGRT in lymphoma management and future directions will be discussed.
Introduction
Image-guided radiation therapy (IGRT) is a process of incorporating imaging techniques during radiation therapy (RT) to improve treatment accuracy. Such integration of imaging allows real-time or near real-time visualization of anatomical information to ensure that the target is in its position as planned. Changes in tumor volume and location due to organ motion during treatment can therefore be compensated. IGRT coupled with intensity-modulated radiation therapy (IMRT) is a very complex process that has evolved slowly over many years paralleled with major advances in functional human imaging, image-registration and fusion techniques [12]. Typically, IGRT employs advanced imaging modalities to localize the target precisely before or during each treatment delivery. As the definition of IGRT is not universally standardized and open to various interpretations, the Radiation Therapy Oncology Group IGRT Committee [3] defines IGRT as radiation treatment design and delivery using modern imaging methods, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and ultrasound (US), in target and non-target structures. It requires accurate and reproducible patient immobilization, knowledgeable selection of IGRT techniques, proper image registrations (rigid/deformable) and accurate clinical definition of disease target margins. IGRT has been gaining popularity and acceptance rapidly in RT over the past 10 years. It has been used increasingly on prostate, bladder, head and neck, and gastrointestinal cancers [456]. However, the role of IGRT in lymphoma management is not well defined as there are only very limited published data currently available. The scope of this paper is to review the current use of IGRT in the management of lymphoma.
Technical Aspects of IGRT
Conventionally, IGRT with on-board imaging can monitor target motion by two-dimensional (2D) orthogonal kilovoltage (kV) X-rays daily [7]. However, it matches only the boney anatomy, and some targets may move independently as most tumors are soft tissue. Currently, some of the most commonly used IGRT techniques with volumetric imaging are the integrated diagnostic CT on rails [8] and megavoltage (MV) or kV cone-beam computed tomography (CBCT) systems [910] which acquire many 2D image projections over the target volume and reconstruct them into a three-dimensional (3D) volume to provide volumetric imaging in less than 5 minutes and allow for radiographic or fluoroscopic monitoring throughout the treatment process to offset day to day set-up variations due to organ motion and physiologic changes (lungs, bowels, rectum and bladder). Such systems also allow monitoring and adjusting for tumor response. The CBCT images are 'matched' with the reference planning CT using image fusion algorithms and adjustments are made as necessary prior to treatment delivery. Fig. 1 demonstrates IGRT with CBCT in a patient with bulky mesenteric diffuse large B-cell lymphoma (DLBCL).
IGRT with CBCT of a 74-year-old man with bulky mesenteric DLBCL. (A) CBCT is matched by fusion with the reference CT before treatment. (B) Alternatively CBCT can be matched by squares with the reference CT (purple, internal target volume; green, CBCT; pink, CT).
Helical tomotherapy (HT) integrates a small MV X-ray source similar to a diagnostic CT scan into the linear accelerator and uses the MV X-rays to create volumetric images of the target within the body in the treatment setup position [11]. Although the contrast resolution of MVCT is somewhat lower than that of the kV CBCT [12], it provides good spatial resolution as another fast IGRT approach that could be done directly to align the patient before treatment delivery.
IGRT with US imaging has been used mostly for soft tissue tumors such as breast and prostate and it can track intrafraction target motion (via trans-perineal imaging for prostate) during daily treatment. US IGRT improves daily treatment positioning accuracy without added ionizing radiation and it requires relatively low cost of maintenance. However, US imaging inherently has higher inter-observer errors because of relatively poorer image quality compared with CBCT [13].
Optical tracking utilizes a special camera to track the target in real-time through visual cues. It relays the patient's position coordinates and compares them with the reference set-up points. Thus, a treatment couch translation is determined that may result in the re-alignment of the patient's position. Intrafraction monitoring of patient position can also be performed by placing an optically tracked object on a region of interest to gain information (including gating) on radiation delivery and to potentially reposition the patient as needed during treatment. Alternatively, some systems integrating optical tracking with in-room imaging devices allow for real-time feedback by imaging the patient directly and tracking the skin surface of the patient [14].
Cine MRI can be integrated into RT machine to provide fast real-time high-resolution images of patient's internal anatomy and it allows tracking of soft-tissue targets during treatment without additional radiation exposure. Potentially, functional information from the diffusion-weighted MRI at mid-treatment may provide measurable data on early response to guide adaptive strategies in RT.
RT using electromagnetic transponder systems (ETS) is technically not IGRT as it entails no 'images.' However, ETS serves the same clinical function as imaging systems to provide continuous analysis of setup error similar to that of the optical tracking devices. Hence, ETS technology is usually classified as an IGRT approach.
In order to compensate for lung movement from respirations, IGRT with respiratory gating has been used to spare more normal lung and cardiac tissue [15]. This is accomplished by four-dimensional (4D) imaging monitoring the patient's inspiration and expiration cycles that allows smaller fields and tighter margins to be used safely. Treatment may be slightly prolonged as treatment is delivered only when the tumor is within the treatment field.
Lymphoma Imaging
IGRT cannot exist without advanced imaging, which plays a pivotal role in the technical and clinical success of IGRT in cancer management. Some of the major contributing factors in accuracy of radiation treatment planning and delivery are the uncertainties in target delineation of disease extent and organ motion during treatment. IGRT incorporates imaging coordinates from the treatment plan to be delivered in order to ensure the patient is properly aligned in the treatment room [16]. As such, IGRT requires accurate delineation, and precise treatment planning and delivery. The recent advances in human imaging have overcome some of the obstacles in delineating the targets and assessing treatment response on post-treatment follow up. Unlike most solid tumors, lymphoma is often not well demarcated with a continuous web-like lymphatic network. Accurate disease assessment is often difficult without good and reliable anatomical and functional imaging.
Both CT and MRI, which have improved acquisition time and resolution significantly over the years, are the mainstay imaging modalities for most cases of cancer management in terms of availability, cost and efficiency [17]. While they may not be very sensitive to detect disease in small lymph nodes (<1 cm), they provide precise anatomical information and can identify mesenteric and splenic disease readily with anatomical correlation. MRI has additional value in detecting disease in bone marrow, the musculoskeletal and central nervous system. However, both modalities rely largely on size criteria to define disease and large treated nodal masses or diffuse visceral involvement frequently can be difficult to interpret by either CT or MRI. Perhaps diffusion-weighted MRI, which can detect small areas of restricted diffusion of water in tumors and appear as bright spots on MRI imaging, has been increasingly used in the tumor evaluation and treatment response assessment for various cancers and may complement the current functional imaging for predicting local treatment response in lymphoma [18192021].
Early targeted lymphatic architectural imaging was accomplished with lymphangiography (LAG), which was pioneered at Stanford in the 1960's [22]. It was a major advance at the time that enabled clinicians to visualize previously undetectable lymphadenopathy in the retroperitoneum. It involves infusing an iodine-containing contrast solution and a dye in the foot lymphatics. The abnormal lymph nodes can then be identified by, not just their sizes, but shape, filling defects, and foaminess. It facilitated the definition of the so-called involved versus extended fields of identified disease and adjacent sites of subclinical involvement. LAG was most valuable for the low-grade non-Hodgkin lymphoma (NHL) subtypes, the great majority (>80%) of which tend to have retroperitoneal involvement. Follow-up LAG helped determine responses to therapy, disease progression, or recurrence. Because the procedure was technically difficult to perform and often painful for patients with prolonged discoloration of the feet, and sometimes secondary infection, it was abandoned gradually in the late 1980's.
Gallium-67 scintigraphy (GS) was an early functional imaging used diagnostically and prognostically in patients with NHL and Hodgkin lymphoma (HL) for over three decades [23]. Gallium-67 (Ga-67), a single-photon emitting agent, is taken up preferentially by active, viable lymphoma cells by binding to transferrin receptors and can be used to monitor treatment response and differentiate between residual viable tumors and residual fibrosis with a relatively high sensitivity and specificity especially for the detection of early recurrence [24]. GS was the best available functional imaging at one time for the follow-up of treatment response and for the assessment of treatment outcome in patients with lymphoma. However, GS has a low spatial resolution and contrast, variable normal tissue physiologic uptake patterns, especially in the bowels, and lack of anatomical landmarks, which makes precise localization of abnormal findings difficult. Its accuracy depends upon the proper injection of high-doses of Ga-67 (296 to 370 MBq/kg of body weight in adults), the use of single-photon-emission computer tomography cameras and acquisition techniques and skilled and experienced interpretation of the images. Imaging with other single-photon emitting tumor-seeking agents has also been used in lymphoma management, including thallium-201, technetium-99m methoxyisobutylisonitrile and indium-111 octreotide [23]. However, they were not superior to GS. Similar to LAG, GS has been largely replaced by the emerging simpler, better tolerated, and less operator-dependent imaging modalities.
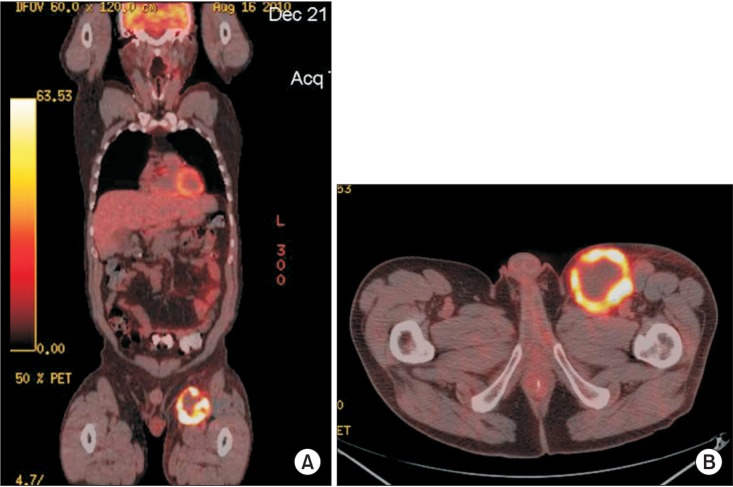
Following the clinical experiences with GS, modern functional imaging methods use positron-emitting agents. Among the several radiopharmaceutical positron-emitting agents (11C-MET, 11C-TYR, 15CO2, 15CO, etc.) currently available [2325], fluorine-18 fluorodeoxyglucose (FDG), the most commonly used agent, is also a viable-tumor seeking agent and shares many similarities with GS as a functional imaging modality independent of morphological criteria. FDG is a radiolabeled glucose analog that is transported into metabolically active cells like regular glucose. Unlike GS that takes up to 14 days in acquisition time, FDG reaches a near equilibrium state in 60 to 70 minutes after injection [26]. As FDG decays, it emits positrons that annihilate with electrons, generating specific 511 keV photons in opposite directions that are detected by either the camera-based or dedicated PET systems. FDG-PET has mostly replaced GS in the evaluation of lymphoma. As it requires a lower dose (3.5 to 8 MBq/kg body weight) and detects metabolically active disease by its increased glycolysis or uptake of FDG, which is proportional to mitotic activity, the degrees of metabolic uptake may help separate high from low-grade tumors with a generally higher uptake in HL and aggressive NHL [262728]. The reported mean maximum standardized uptake values (SUV) for nodular sclerosing HL, mixed cellularity HL, and nodular lymphocyte-predominant HL are 16.3, 20.8, and 9.3 respectively [29]. For NHL, there is a slight overlap of SUV between aggressive and indolent histology with a mean SUV of 19.6+/-9.3 and 7.0+/-3.1, respectively [28]. Although the PET sensitivity and specificity vary in different situations, it has made significant clinical impacts in tumor diagnosis, staging, and treatment response assessment [30]. The presence of persistent interim tumor FDG-uptake is a significant negative prognostic factor in patients with HL and aggressive NHL [2931]. In a meta-analysis with 854 patients, the median sensitivity, specificity, and false positivity for detection of lymphoma were 90%, 90% and 10%, respectively [32]. However, very small diseases (<5 mm) may still not readily be detected. Other drawbacks include the inherent subjectivity of interpretation, false positivity due to increased uptake in metabolically active inflammatory tissues from infection, surgery, trauma, or sarcoid and other non-malignant inflammatory conditions [33]. Nevertheless, PET-CT combines functional and anatomical imaging in a single device to provide simultaneous metabolic and structural information. Radiation treatment planning integrated with PET can use soft-tissue anatomical images with superimposed functional images that help identify metabolically active sites [34]. Fig. 2 demonstrates anatomically correlated intense uptake in the left inguinal nodes in a young patient with isolated recurrent DLBCL.
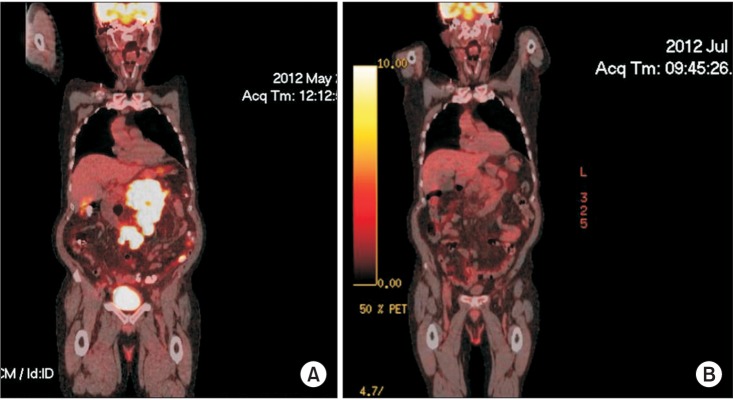
In a study of 135 patients with clinical stages I/II supradiaphragmatic HL treated with chemotherapy and involved-field to involved-node radiation therapy (INRT), the addition of pre-chemotherapy FDG-PET to CT helped identify additional FDG-avid lymph nodes and led to a clinical target volume (CTV) increase in 60% of the patients [35]. The mean increases in the gross tumor volume (GTV) and CTV were 8.8% and 7.1%. PET-CT leads to more accurate INRT delineation. It allows target adjustment due to volume changes in response to chemotherapy (adaptive planning) during treatment. More importantly, PET-CT provides excellent post-treatment evaluation, especially in patients with HL and DLBCL [3637]. Although the clinical benefits may be less apparent for other lymphomas, PET-CT is considered the state-of-the-art imaging technique and the most sensitive and specific functional imaging currently available for the assessment of lymphoma and many other malignancies [26]. The current National Comprehensive Cancer Network guidelines version 2.2015 have incorporated the use of FDG-PET/CT into initial staging and restaging as the response criteria after chemotherapy for both HL and NHL in specific situations [38]. Briefly, baseline PET-CT is recommended for lymphomas that are potentially curative such as HL and DLBCL. It may be used to exclude systemic disease in clinically localized lymphoma, monitor progress of therapy and evaluate residual masses after chemotherapy. Fig. 3 shows the pre-treatment PET-CT of a patient with bulky mesenteric DLBCL. At mid-treatment after 3 cycles of systemic chemotherapy, PET-CT showed dramatic resolution of the mass and hypermetabolic activity. Such early therapy response at mid-treatment with metabolic imaging may predict better outcome of patients with aggressive lymphoma and help modify subsequent therapy as necessary [2639].
The Current Role of IGRT and Image-Guided Adaptive Radiation Therapy in Lymphoma Management
Lymphoma is a heterogeneous and complex group of tumors with a wide range of presentations. The treatment and prognosis can be influenced by multiple interacting factors, some of which include the histology, co-morbidities, International Prognostic Index, disease burden, number of disease sites and response to initial therapy [40414243]. For lymphomas in general, RT has been consistently confirmed as the most effective single modality for local control and an important component of combined therapy for most patients. RT has been used as definitive therapy, combination treatment, salvage therapy and palliation for lymphoma [4445]. However, there are supporting data in both HL and NHL that traditional radiation doses are higher than necessary for disease control and related to the incidence of late effects [4647]. Identifying the optimal radiation dose based on the initial disease extent and response to chemotherapy is emerging. The current general guidelines by the International Lymphoma Radiation Oncology Group (ILROG) [48] and the Lymphoma Radiotherapy Group of UK [49] recommend a dose of 30 Gy or less for HL and aggressive NHL and 24 Gy for indolent lymphomas; a lower dose of 20 Gy in combination therapy for early-stage low-risk HL lymphoma may be sufficient. For residual lymphoma after chemotherapy, a dose 36 Gy may be considered. Depending on the primary sites and concurrent therapies, primary extranodal lymphomas, and bulky diseases may require higher doses [5051]. While not all patients with lymphoma will benefit from consolidative RT in the present era of advanced chemotherapy and immunotherapy, recent reviews of literature support that the current standard of care for many patients with DLBCL is multimodality approach, which involves a combination of chemotherapy, immunotherapy, and local radiotherapy [52]. Such approach has yielded very high local control and survival rates. Even in the era of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), those patients who receive consolidative RT have a significantly better local control, event-free survival (EFS) and overall survival (OS) including a subset of patients who have achieved a complete response to chemotherapy [4453]. A recent meta-analysis of four qualified retrospective studies (633 patients) showed that consolidative RT after complete response to R-CHOP improved OS and EFS in all patients compared with no RT [54]. Similarly, for patients with stage III Hodgkin lymphoma, Phan et al. [55] reviewed 118 patients who received consolidative RT after complete response to ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). On multivariate analysis, mediastinal consolidative RT was associated with improved DFS and OS.
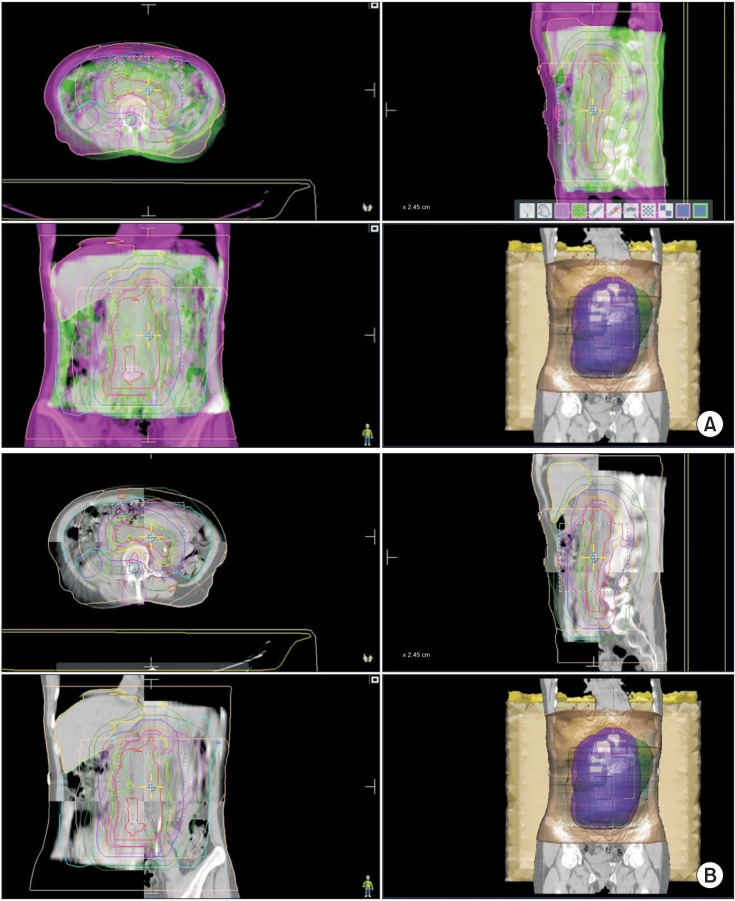
The trend in field size reduction from IFRT has been addressed in the literature [35]. The ILROG Steering Committee recently published consensus guidelines on the use of RT in nodal and extranodal NHL and HL in the modern era of combined modality treatment and endorsed the highly conformal INRT for patients for whom optimal imaging is available and introduced a new concept of involved-site radiotherapy (ISRT) as the standard conformal therapy, when optimal imaging is not available [48]. The difference between INRT and ISRT depends on the quality and accuracy of the pre and post-chemotherapy imaging which determines the margins needed to allow for uncertainties in the contouring of the target volumes. The target volumes are based on detectable tumor extension at presentation and at completion of chemotherapy, using contrast-enhanced CT, PET, MRI, or a combination of these techniques. Treatment planning is based upon CT with 3D definition of volumes and treatment delivery is accomplished with advanced techniques, such as IMRT and IGRT. The International Commission on Radiation Units and Measurements concepts of GTV, CTV, internal target volume (ITV), and planning target volume (PTV) are used for defining the target volumes [56]. In brief, with a few clinical exceptions, the GTV is based on the PET-defined pre-chemotherapy sites of involvement and includes the involved nodes (or organ) and any residual disease, not extending into air, muscle planes or bone, unless the muscle or bone invasion is present. Some investigators use the PET imaging to determine not just the gross tumor volume but also the biological target volume (BTV) to include imaging data specific to tumor biology beyond that provided by anatomical imaging alone [57]. The CTV should be defined by direct 3D volumetric expansion of GTV in the craniocaudal direction of lymphatic spread by 1.5 to 2 cm, constrained to tissue planes and air cavities. The margin allows for uncertainties in PET resolution, image registration and changes in volume since imaging, patient positioning and potential subclinical spread patterns of the disease and adjacent organ constraints. ITV is mostly relevant when the CTV is moving internally, like targets within the chest and upper abdomen, with respiratory movements and is defined as the CTV plus a margin determined by 4D simulation CT or alternatively, by fluoroscopy, to account for motion-related uncertainties in size, shape, and position of the CTV. Thus, ITV is formed by fusion of multiple CTVs at various phases of respiration and margins of 1.5 to 2 cm in the superiorinferior directions may be necessary. Gastric lymphoma, for example, may move internally and will benefit from IGRT with ITV delineation. Fig. 4 shows a 51 year old man with H. pylori negative gastric mucosa-associated lymphoid tissue lymphoma stage (MALT) lymphoma stage IEA who was treated to 3,600 cGy in 20 fractions with daily CBCT verification. ITV was obtained with 4D CT scan and then PTV expansion was performed. ITV or CTV to PTV margins are more variable and subjective. In general, it can range from 0.5 to 1.0 cm, depending upon the technique, setup accuracy and degrees of internal organ motion and should be determined individually for each disease site and treatment center [58]. Fig. 5A illustrates how much IGRT coupled with IMRT and respiratory gating helped protect this patient's heart from consolidation RT to the upper para-aortic area and spleen after complete response to chemotherapy for DLBCL. Fig. 5B demonstrates beautiful separation between the heart and spleen with inspiration. Fig. 5C is the resultant dose-volume histogram with minimal dose to the heart.

Gastric MALT lymphoma stage IEA. Internal target volume was obtained with 4D CT scan and planning target volume expansion was performed.
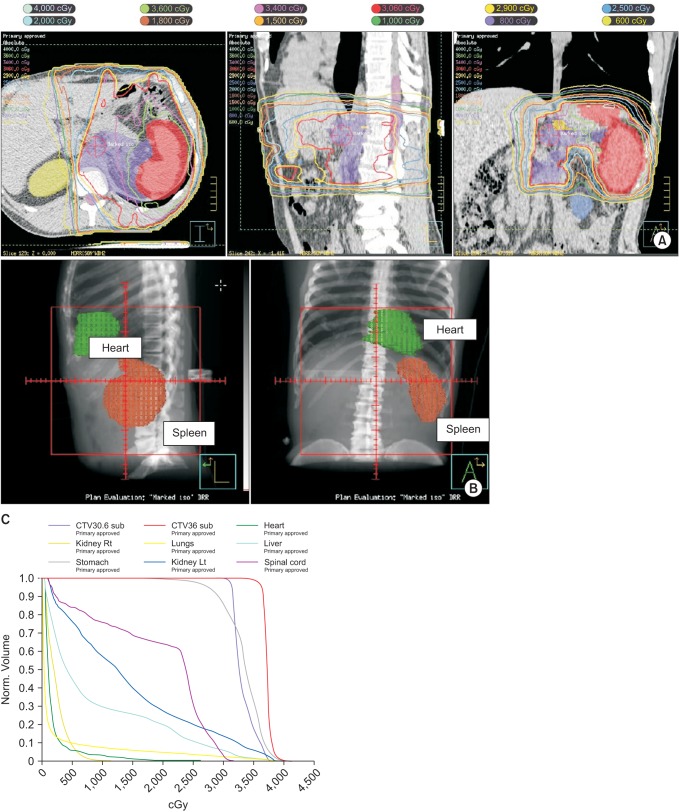
A patient treated with consolidation radiation therapy to the upper para-aortic area and spleen after complete response to chemotherapy for DLBCL. (A) IGRT-IMRT and respiratory gating show very conformal isodose lines around the target with sparing of the heart and kidneys. (B) Respiratory gating takes advantage of increased separation between the heart and spleen with inspiration. (C) Dose-volume histogram shows minimal dose to the heart and other organs at risk.
Clinical Aspects of IGRT
Despite the increasing use of IGRT/IMRT in patients with lymphoma, its impact on clinical outcomes remains to be confirmed. Currently, there are no prospective randomized trials to compare the clinical benefits of IGRT with that of conventional RT in lymphomas. A few anecdotal cases of lymphoma treated with IGRT have been reported with good results [596061]. Tomita et al. [62] conducted a pilot comparison of radiation treatment plans between the IGRT with HT-IMRT, and conventional 3D-conformal radiation therapy (3D-CRT) for eight patients with nasal natural killer/T-cell lymphoma using the parameters of the target coverage and homogeneity for the PTV and the maximum and mean doses for organs at risk. HT-IMRT achieved significantly better PTV coverage than 3D-CRT did, with more than 99% of the PTV receiving 90% and 95% of the prescribed dose vs. 89.1% and 84.5% for 3D-CRT, and equivalent or slightly better organ at risk avoidance. The homogeneity index was 0.29 for IMRT and 0.046 for 3D-CRT.
Chargari et al. [63] reported their preliminary clinical experience of IGRT using HT in 6 consecutive, previously heavily treated patients with refractory bulky residual malignant lymphoma referred for salvage before stem cell transplant. The patients received 30 to 40 Gy with involved fields. Treatment was very well tolerated by most patients with no grade 2 or higher toxicity. All but one patient experienced complete clinical, radiologic, and metabolic remission after HT. The authors attributed the favorable outcomes to more effective sparing of critical organs with HT, which is particularly relevant in heavily treated patients. Doses to the heart, lungs, esophagus, and parotids were significantly decreased.
In a retrospective analysis of 90 patients with stage IIA HL involving the mediastinum who had a complete response to chemotherapy, Filippi et al. [64] compared 41 patients treated with 30 Gy involved-site IGRT-IMRT with 49 patients treated with involved-site 3D-CRT. At a median follow-up of 54 months for 3D-CRT and 24 months for IGRT-IMRT patients, there were no differences in relapse-free survival, 98.7% vs. 100%. However, IGRT-IMRT patients showed a significantly lower incidence of grade 2 acute toxicity (mainly mucositis), 9.8% vs. 32.7%. As this was a retrospective study, the authors acknowledged the shorter follow-up in IGRT-IMRT group that may have influenced the outcomes. Nevertheless, IGRT-IMRT helps improve treatment delivery accurately and minimize normal tissue being treated.
A recent publication has summarized some of the specific clinical and technical aspects of IGRT in the management of lymphoma [65]. As we continue to strive to lower the side effects by adapting or reducing the treatment margins or volume during radiotherapy, IGRT-IMRT with functional imaging is necessary to provide real-time monitoring of tumor response and allow re-delineation, re-planning and reoptimization, especially in the treatment of large and bulky radiosensitive lymphomas. Implementation of reduction in both treatment volume and overall treatment dose is expected to minimize the risks of acute and late sequelae significantly while still maintaining the excellent local control of disease. However, individualized treatment with an integrated multidisciplinary approach is still the key for the optimal outcome for patients with lymphoma. Blair and Sharma [66] recommend the following main IGRT principles that should apply to lymphoma management: 1) both pre-treatment and post-treatment PET or PET/CT should be obtained for patients undergoing chemotherapy regimens prior to receiving RT. Treatment planning considerations include factors such as: the apparent degree of involvement of all clinically involved nodal and extranodal groups, the degree and rapidity of response to chemotherapy, the proximity of nearby critical normal tissues and the presence of post-chemotherapy residual FDG avid tissues or FDG-negative 'scar' tissue demonstrated on CT or MRI; 2) deliberately inhomogeneous dose delivery strategies with IMRT methodologies where warranted by normal tissue proximity will result in highly conformal dose delivery with minimization of normal tissue margins; 3) appropriate immobilization using templates, masks, positioning rigs, etc.; and 4) serial re-evaluation during the course of treatment to determine whether registration coordinates have moved or if 'adaptive' re-planning or re-calculation of absorbed doses is warranted.
Conclusion and Future Directions
Combining advanced volumetric imaging technology with precise IGRT techniques to localize and treat tumors can improve safety and accuracy, especially in target sites that are prone to movement, such as those located in the chest and abdomen and those close to adjacent critical organs. A multimodality approach with the application of IGRT principles and modern functional imaging techniques helps improve staging, treatment delivery and response assessment. Treatment field margin reduction and dose escalation, if necessary, are achievable, while toxicities are minimized. PET-CT will continue to aid in target definition, treatment planning, tailoring of intervention, and early detection recurrence of tumor. As functional imaging continues to improve, the concept of automated target volume delineation in IGRT treatment planning using FDG-PET/CT may help improve objectivity in a better-defined BTV [67]. Deformable registration is also emerging and being utilized in IGRT to improve target acquisition and localization [6869]. As technologic advances continue to evolve and gain momentum in lymphoma management, successful adoption of these technologies requires clear understanding of the complexity of IGRT, current knowledge, quality assurance, necessary training and skills associated with such implementation in order to exploit the benefits of IGRT. It is likely that the full benefits of the IGRT paradigm will slowly be realized and more clinical outcomes data will eventually emerge. Ultimately IGRT will have to prove itself in effectiveness, efficiency, long-term clinical outcomes, and financial impacts in the management of lymphoma.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.