Set-up errors in head and neck cancer treated with IMRT technique assessed by cone-beam computed tomography: a feasible protocol
Article information
Abstract
Purpose
To investigate set-up errors, suggest the adequate planning target volume (PTV) margin and image-guided radiotherapy frequency in head and neck (H&N) cancer treated with intensity-modulated radiotherapy (IMRT) assessed by kV cone-beam computed tomography (CBCT).
Methods
We analyzed 360 CBCTs in 60 patients with H&N cancer treated with IMRT. The target delineation was contoured according to ICRU62. PTVs were generated by adding a 3–5 mm margin in all directions to the respective clinical target volumes. The kV CBCT images were obtained at first three days of irradiation and weekly thereafter. The overall mean displacement, range, systematic (∑) and random (σ) errors were calculated. Adequate PTV margins were calculated according to the van Herk formula (2.5∑ + 0.7r).
Results
The mean of set-up errors was less than 2 mm in any direction. The overall frequency of set-up displacements greater than 3 mm was 3.9% in medial-lateral (ML) direction, 8% in superior-inferior (SI) direction, and 15.5% in anterior-posterior (AP) direction. The range of translations shifts was 0–9 mm in ML direction, 0–5 mm in SI direction and 0–10 mm in AP direction, respectively. After systematic set-up errors correction, the adequate margin to overcome the problem of set-up errors was found to be less than 3 mm.
Conclusion
Image-guided kV CBCT was effective for the evaluation of set-up accuracy in H&N cancer. The kV CBCT at first three fractions and followed-by weekly appears adequate for reducing significantly set-up errors in H&N cancer treated with IMRT technique. Finally, 3–5 mm PTV margins appear adequate and safe to overcome the problem of set-up errors.
Introduction
In Western countries head and neck (H&N) cancer accounts for about 5% of all tumors. Squamous cell carcinoma is the most common histotype reaching about 90%, and generally arises from the mucosa of the upper aerodigestive tract [1,2].
The etiology of these tumors has traditionally been related to tobacco and alcohol consumption, whereas in the last decade infection from the human papilloma virus has been identified as an emerging cause [3]. It is estimated that about 70% of H&N cancer at the diagnoses consist in locally advanced head and neck cancer (LAHNC). According to the international guidelines, definitive radiotherapy (RT) plus platinum-based chemotherapy or cetuximab is the standard treatment in these patients [4-6].
In the last few decades, due to technological improvements in treatment such as intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT), the use of radiotherapy in the H&N cancer has significantly improved both in curative and adjuvant RT treatment [7-9].
IMRT is a highly conformal radiation technique that allows delivery of high, curative radiation doses to the gross tumor, lymph nodes, and high-risk areas while sparing the adjacent organs.
For this reason IMRT is ideal for the treatment of H&N cancer and recent studies showed that IMRT can potentially improve local–regional control, reduce side effects especially xerostomia, and improve quality of life [10-16].
The improved dose conformality and the possibility to delivery high, curative radiation doses to the gross tumor achieved with these new techniques requires greater accuracy in treatment planning and during the course of RT due to reduced target delineation uncertainty and set-up errors.
The most significant errors in clinical RT are usually caused by set-up and anatomic variations during the treatment course due to tumor shrinkage, weight loss and/or organ motion. Set-up errors are defined as any deviation (measured in millimeter or in degree) of the patient position during any fraction of treatment compared to the reference patient position at the planning computed tomography (CT) scan.
Conventionally, set-up verification has been done with the acquisition of 2D kilovoltage (kV) or megavoltage (MV) portal images, which can be compared or matched with the digitally reconstructed radiographs (DRR) generated from the planning CT scan. The recent development of volumetric imaging techniques such as cone-beam computed tomography (CBCT) deeply impacted the overall quality of IGRT, moving from 2D verification of the position of bony landmarks to 3D assessment of the position of target volumes and of organ at risk [17-19].
The aim of our study was to investigate set-up errors, suggest the adequate planning target volumes (PTVs) margins and IGRT frequency in H&N cancer treated with IMRT technique assessed by kV CBCT.
Materials and Methods
1. Patient and tumor characteristics
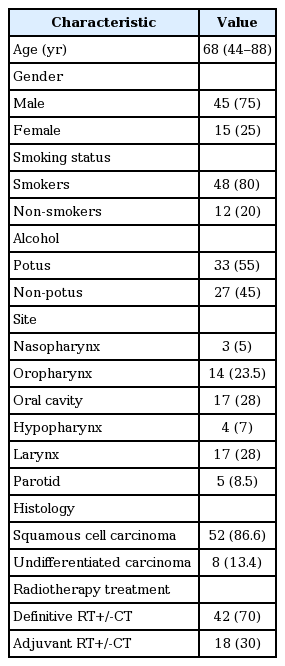
Between September 2014 and September 2015, 360 CBCT scans of 60 patients affected by histologically confirmed H&N cancer who were treated with the IMRT technique were analyzed. The majority of patients treated were male (75%) and only 25% were female. The median age was 68 years with a range from 44 to 88 years. Regarding the histology examinations, the majority of patients (86.5%) exhibited squamous cell carcinoma and 13.4% of the examinations yielded undifferentiated carcinoma. The types of H&N cancer treated were larynx (28%), oral cavity (28%), oropharynx (23.5%), hypopharynx (7%), nasopharynx (5%), and parotid cancer (8.5%). Of the total patients treated, 42 patients (70%) underwent curative chemoradiotherapy treatment and 18 patients (30%) were treated with adjuvant intent after surgery.
Concurrent platinum-based chemotherapy was administered when clinically indicated according to international guidelines [4,5]. In details, chemotherapy was prescribed (if not contraindicated) in all patients with LAHNC underwent curative radiotherapy treatment and in patients with major risk factors after surgery (such as positive margins and extracapsular extension). Chemotherapy was given weekly using cisplatin 40 mg/m2 intravenous (IV) over 1 hour during the 6-week RT course for a maximum of 6 cycles, or cisplatin 100 mg/m2 IV once every 3 weeks for a maximum of 3 cycles. Patients and tumor characteristics are summarized in Table 1.
2. Simulation, immobilization system and treatment planning
All patients underwent planning CT simulation on supine position on a GE LightSpeed RT 16 CT simulator (GE Healthcare, Waukesha, WI, USA) using 2.5 mm slice thicknesses and contrast medium injection due to better definition of gross tumor volumes (GTVs) and clinical target volumes (CTVs). We utilized a head-shoulder thermoplastic mask (Klarity Green; Klarity Medical Products, Newark, OH, USA) as immobilization system. The use of a thermoplastic head and shoulder immobilization device makes the set-up procedure easily reproducible allowing the detection of systematic errors with a low number of image-guided fractions [20]. The position of room lasers was marked on the mask and markers were put on the laser crossings to define a reference point according to tumor localization and to the volume to be treated.
The CT data sets were transferred to the Focal and Varian Eclipse treatment planning system through DICOM network. The target delineation was performed by a radiation oncologist according to ICRU62; the PTVs were generated to compensate for geometrical uncertainties by adding an isotropic 3–5 mm margin in all directions to the respective clinical CTVs [21]. A GTV-Tumor/GTV-Nodal was contoured in all cases of curative treatment. The CTV was delineated around the GTV to cover areas at risk for the presence of microscopic disease. A margin of 3 mm was added around the spinal cord and the brainstem to generate a planning at risk volume. According to international guidelines, the prescribed doses were as follow: 66 Gy (delivered in 30 fractions; 2.2 Gy/fraction) for high risk PTVs, 60–63Gy (delivered in 30 fractions; 2–2.1 Gy/fraction) for intermediate risk PTVs, and 54 Gy (delivered in 30 fractions; 1.8 Gy/fraction) for low risk PTVs [4,5,19,20].
The RT treatment was performed with IMRT technique. The IMRT plans were created on the Varian Eclipse treatmentplanning system using coplanar beams with 6 MV photons and the treatment was performed with DHX LINAC, VARIAN System. Before the treatment all patients were re-positioned in the treatment room aligning the signs marked on the mask with room lasers. If necessary, couch shifts were applied according to planning indications to reach the treatment isocenter, aligning the signs marked on the mask with room lasers.
3. Image guidance procedures and set-up errors analyses
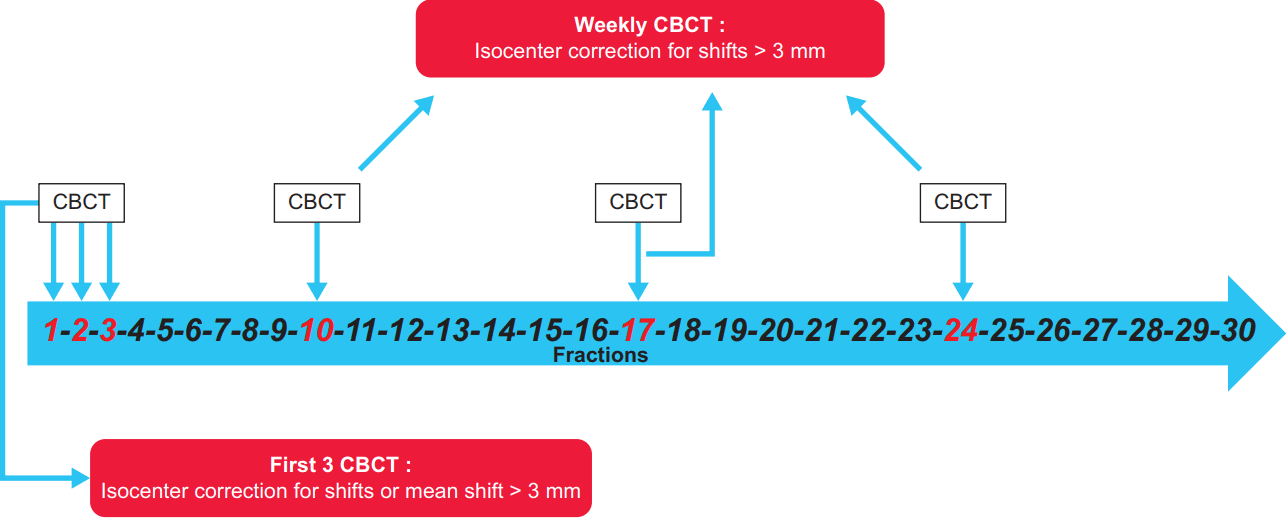
In all patients pretreatment kV CBCT images were obtained at first 3 days of irradiations and set-up error corrections were made before treatment if the translational set-up error was greater than 3 mm in any direction. Subsequently a weekly kV CBCT was repeated for whole duration of treatment and if translational shifts in any directions were greater 3 mm were corrected.
One CBCT image acquisition was not repeated after correction or after treatment delivery. Image registration was usually performed by an automatic algorithm applied to an extensive region of interest (clip-box) encompassing the whole PTV.
If necessary offline view was utilized to calculate the translation shifts and data extraction. Observed translational displacements in three axes—medial-lateral (ML), superior-inferior (SI), anterior-posterior (AP)—were recorded and always corrected online before delivering treatment.
The entire procedure, starting from the patient set-up to the beginning of treatment, took approximately 6 minutes. Quality assurance procedures for CBCT images included a monthly check of mechanical system and image quality, as well as weekly geometrical accuracy tests to verify that the CBCT reconstruction center was coincident with the isocenter of the linear accelerator.
Also, the mean value of the first three CBCTs recorded in each of three axes was calculated and in case of a mean error greater than 3 mm a systematic set-up correction (modifying laser alignment signs on the mask) was performed. One CBCT image acquisition was repeated after correction or after treatment delivery.
Fig. 1 describe the summary diagram used in our center due to minimize set-up errors through CBCT in H&N cancer treated with IMRT.
4. Statistical analysis
Before performing the analysis, an exploration phase was carried out. Categorical data were described by frequency and percentage, whereas continuous data were done by mean, median, and range.
All errors entered and analyzed separately for each direction (ML, SI, AP). For each patient, the mean and standard deviation (SD) of all recorded errors were calculated. Systematic error (Σ) stands for the overall mean (M) calculated as the average value of all individual means, measuring the overall accuracy of disease-specific set-up procedures. The SD of the group systematic error (Σ) was calculated as the SD of the individual means. The overall indicator of the group random error (σ) was calculated as the root mean square of the individual SD of all patients.
Finally, for the calculation of the margin to be added to CTV to obtain PTV we used the van Herk formula (2.5Σ + 0.7r) [22] which ensures that 90% of the doses is given a CTVs of at least 95% of the prescribed dose.
Analyses were performed using the SPSS version 22 software (IBM, Armonk, NY, USA).
Results
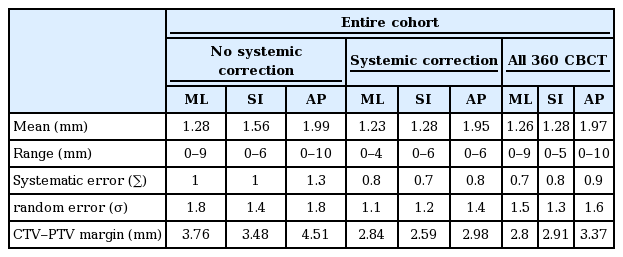
A total of 360 CBCT scans in 60 patients affected by H&N cancer treated with the IMRT technique were acquired and analyzed. The mean ± SD of set-up errors was 1.26 ± 1.5 mm in the ML direction, 1.28 ± 1.3 mm in the SI direction and 1.97 ± 1.6 mm in the AP direction.
Table 2 reports the analysis of the displacement of set-up errors both with and without systematic correction; the ranges of translational shifts were 0–9 mm in the ML direction, 0–5 mm in the SI direction, and 0–10 mm in the AP direction. The overall frequencies of setup displacements greater than 3 mm were 3.9% in the ML direction, 8% in the SI direction and 15.5% in the AP direction (Table 2).

Frequencies of CBCT translational shifts greater than 3 and 5 mm in ML, SI, AP directions with and without the application of the systematic correction protocol
When the CBCT scans were analyzed before systematic corrections, the frequencies of the setup errors greater than 3 mm were 17.8%, 10.6% and 5.6% in the AP, SI, and ML directions, respectively. After set-up error correction (i.e., corrections via couch shifts or patient repositioning) these rates were reduced to 13.3%, 7.2%, and 2.2% in the ML, SI, and PA directions, respectively.
In Fig. 2A–C, the scatter-plots of translational displacements in three axes (ML, SI and AP) with or without the application of the systematic corrections are reported. Additionally, overall set-up displacements in any direction that were greater than 5 mm and which consequently required an online shift correction were present in 5.3% of the 360 CBCT scans analyzed (19/360).
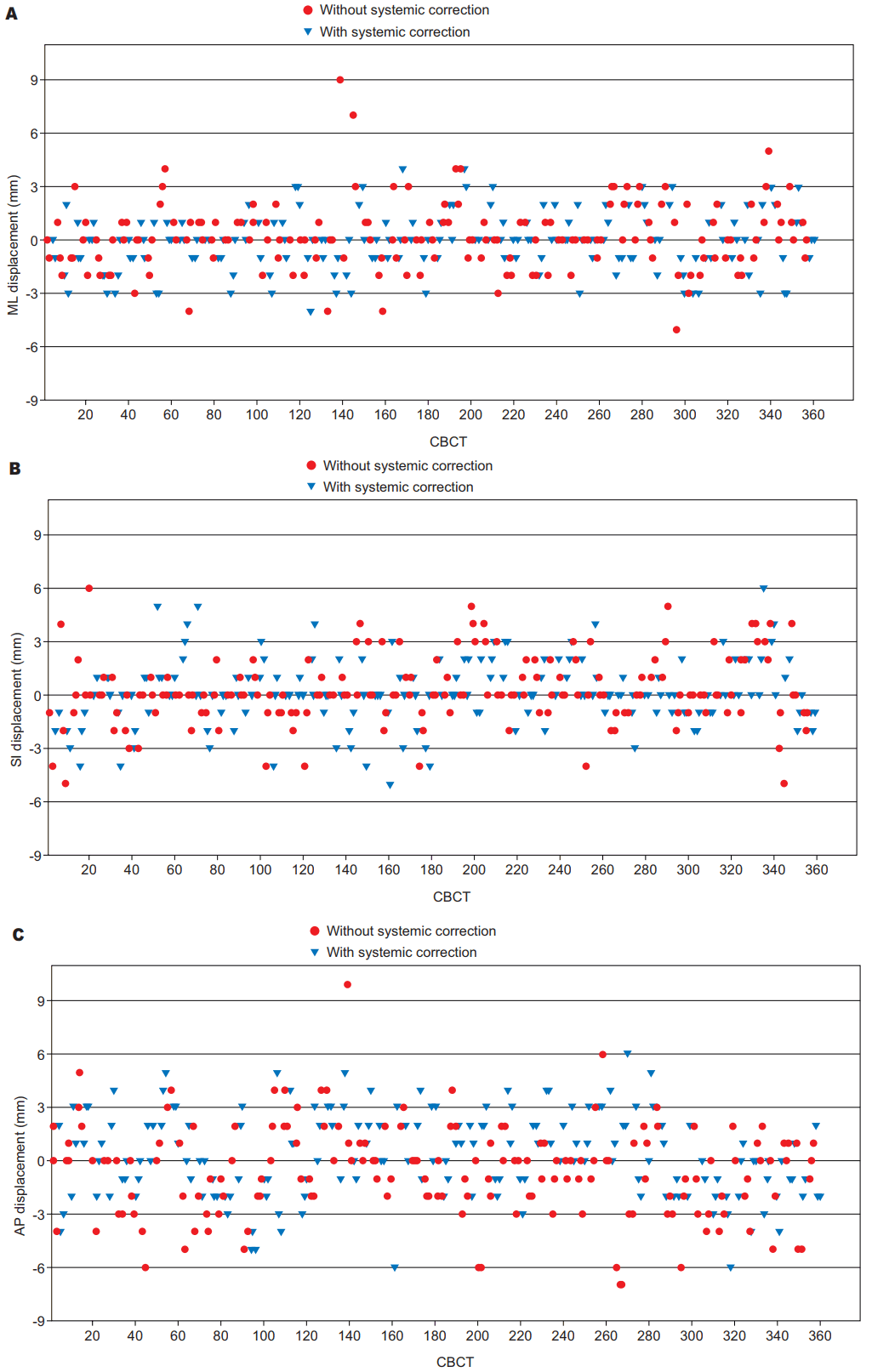
Scatter-plot of translational displacements in the three axes without and with the application of the systematic correction protocol: (A) ML direction, (B) SI direction, and (C) AP direction. CBCT, cone-beam computed tomography; ML, medial-lateral; SI, superior-inferior; AP, anterior-posterior.
Finally, according to the van Herk formula, taking a margin of 3–5 mm in all directions from CTVs to obtain the respective PTVs was adequate to overcome the set-up error problem; after the correction of systematic set-up errors, this margin was less than 3 mm (Table 3).
Discussion and Conclusion
Recent studies demonstrated that the IMRT technique enables the delivery of high, curative radiation doses to the gross tumor, lymph nodes and high-risk areas while sparing the adjacent organs [8-11]. For these reasons, IMRT is the ideal technique for the treatment of H&N cancer because it can potentially improve local–regional control, reduce side effects (especially xerostomia) and improve quality of life [12-16].
The improved dose conformality and the possibility of delivering high-radiation curative doses to the gross tumor achieved with IMRT requires greater accuracy in treatment planning and registration during the course of RT, due to reduced target delineation uncertainty and set-up errors.
Several studies have investigated set-up errors in H&N cancer, analyzing the required number (N) of first fractions to be imaged in order to detect systematic errors [23-42]. They also reported that weekly imaging after the first set of verifications was effective in further reducing the value of N while achieving the same accuracy [28].
The study of Houghton et al. [29] evaluated different imaging strategies in H&N cancer. They demonstrated that the systematic displacement registered after 3 fractions correlated well with the mean error of the other fractions delivered, and no additional benefit was noted when the mean error from the first 5 fractions was considered.
In our center, we applied a protocol that provides three CBCT scans in the first 3 fractions with the correction of set-up errors greater than 3 mm in any direction, followed by weekly CBCT scans.
Based on the results of our study, a margin of 3–5 mm and weekly CBCT scans after the first three CBCTs on day 1, 2, and 3 of irradiation can reduce significant set-up errors during the RT treatment.
As shown in Table 2, of the 360 CBCTs analyzed in our study only 28% of cases required correction (exhibited shift displacement greater than 3 mm). In particular, analyzing the CBCT scan before set-up error correction yielded a frequency of set-up displacements greater than 3 mm of 34.5%. After set-up error corrections were applied (corrections via couch shifts or patient repositioning) this rate was reduced to 21%.
Furthermore, set-up displacements greater than 5 mm in any direction had a frequency of 7.8% before set-up error corrections were applied, and these rates were reduced to 2.8% after set-up error correction.
As shown in Table 3, based on the van Herk formula (2.5Σ + 0.7r), the margin of 3–5 mm added from CTVs to PTVs was sufficient to overcome the set-up errors in H&N cancer.
Moreover, after the correction of systematic set-up errors, a 3-mm margin was sufficient to overcome the problem of set-up errors.
Similar finding were reported by Dionisi et al. [30], where a total of 420 CBCT scans of patients with H&N cancer were analyzed. A systematic correction was necessary in 43% of patients and the overall value of mean displacement was less than 1 mm in all directions. The PTV margins calculated after online correction were less than 2.5 mm in all directions. The authors concluded that a margin of 5 mm added to CTVs to obtain the respective PTVs was safe in order to overcome the problem of set-up errors, and in particular situations, such as re-irradiation, the close proximity of organs at risk and high-dose regions or IGRT, these margins can be reduced to 3 mm.
Moreover, in 2015, Xu et al. [37] reported the data of a prospective study which investigated the set-up errors in 30 patients affected by nasopharyngeal cancer treated with IMRT, based on weekly CBCT evaluation. Each patient had a weekly CBCT scan before radiation therapy. In the entire study, 201 CBCT scans were analyzed and the author concluded that adding a margin of 3 mm in all directions from the CTVs to obtain the respective PTVs was adequate to overcome the problem of set-up errors. Finally, Velec et al. [38], in a prospective study, compared the intrafraction and interfraction set-up errors in two different thermoplastic masks in patients affected by H&N cancer treated with IMRT. The authors evaluated 762 CBCT scans and the set-up errors before and after corrections were less than 3 mm in any direction. There was no statistical significance between the two different thermoplastic masks, with respect to interfraction and intrafraction set-up errors.
In conclusion, the results of our study confirmed that image guided kV CBCT is effective in evaluating set-up accuracy in H&N cancer patients. This study suggested that kV CBCT at the first 3 fractions and subsequently once a week seems adequate to overcome the problem of set-up errors in H&N cancer treated with the IMRT technique. Finally, adding a margin of 3–5 mm in all directions to the CTVs to obtain the respective PTVs is adequate and safe to overcome the problem of setup errors. In particular situations or in the case of IGRT, these margins can be reduced to 3 mm.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors declare that the preliminary results has been presented at European Society for Radiotherapy and Oncology 35 (ESTRO 35) congress on section “Ensuring quality in head and neck treatment, OC-0274” held in Turin, Italy from April 29 to May 3, 2016.