Prognostic value of FDG PET/CT during radiotherapy in head and neck cancer patients
Article information
Abstract
Purpose
To evaluate the prognostic value of 18F-fluorodeoxyglucose positron-emission tomography (FDG PET) with computed tomography (CT) before and during radiotherapy (RT) in patients with head and neck cancer.
Methods
Twenty patients with primary head and neck squamous cell carcinoma were enrolled in this study, of whom 6 had oropharyngeal cancer, 10 had hypopharyngeal cancer, and 4 had laryngeal cancer. Fifteen patients received concurrent cisplatin and 2 received concurrent cetuximab chemotherapy. FDG PET/CT was performed before RT and in the 4th week of RT. The parameters of maximum standardized uptake value, metabolic tumor volume, and total lesion glycolysis (TLG) of the primary tumor were measured, and the prognostic significance of each was analyzed with the Cox proportional hazards model.
Results
Higher TLG (>19.0) on FDG PET/CT during RT was a poor prognostic factor for overall survival (OS) (p = 0.001) and progression-free survival (PFS) (p = 0.007). In the multivariate analysis, TLG during RT as a continuous variable was significantly associated with OS and PFS rate (p = 0.023 and p = 0.016, respectively). Tumor response worse than partial remission at 1 month after RT was another independent prognostic factor for PFS (p = 0.024).
conclusions
Higher TLG of the primary tumor on FDG PET/CT during RT was a poor prognostic factor for OS and PFS in patients with head and neck cancer.
Introduction
Cancers of the head and neck are relatively rare, accounting for about 3% of all malignancies [1]. Nearly 60% of patients with head and neck cancer present with locally advanced but non-metastatic disease. For locally advanced or unresectable head and neck cancer, radiotherapy (RT) with or without chemotherapy plays an important role. A meta-analysis of chemotherapy in head and neck cancer patients with locally advanced disease revealed that concurrent chemoradiotherapy (CCRT) improved 5-year overall survival (OS) compared with RT alone [2]. Cisplatin is the systemic agent of choice in combination with RT, but cetuximab, an anti-epidermal growth factor receptor 1 monoclonal antibody, can also be used for treatment [3,4].
Although CCRT improves survival, it increases the rate of acute or chronic toxicities compared with RT alone. A large number of head and neck cancer patients receiving CCRT suffer from acute toxicities, and some experience toxicity-related treatment delay or hospitalization [5]. After receiving CCRT for locally advanced head and neck cancer, locoregional recurrence rates are 30%–50% and distant metastasis rates range from 15% to 20% [6,7]. The likelihood of successful salvage treatment for recurrent disease is low. Therefore, it is necessary to determine which subgroup of head and neck cancer patients do not benefit from CCRT or RT and have a high probability of disease relapse. The early identification of these patients would enable the selection of appropriate treatment.
18F-fluorodeoxyglucose positron-emission tomography (FDG PET) combined with computed tomography (CT) has higher sensitivity than FDG PET or CT alone for detecting primary tumors in the head and neck [8,9]. It is not only useful for staging and RT planning of head and neck cancer, some of its metabolic parameters are known to have prognostic value as well [10,11]. Recent studies have assessed metabolic changes in FDG PET/CT during treatment as a method for predicting tumor response to therapy and survival outcomes [12,13].
There are many reports on the prognostic value of FDG PET/CT before or after RT. However, relatively little data are available on the predictive value of FDG PET/CT during RT for head and neck cancer. The aim of this study was to perform quantitative evaluation of the ability of FDG PET/CT parameters to predict the treatment outcomes of head and neck cancer patients, in scanning performed before and during RT.
Materials and Methods
1. Patients and treatment methods
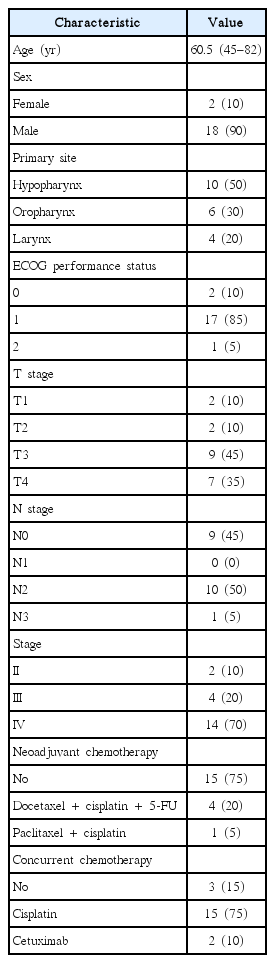
Enrolled in this prospective study were 20 patients with squamous cell carcinoma of the head and neck who were treated at Seoul National University Boramae Medical Center between March 2012 and March 2014. Head and neck cancer patients who received definitive RT or chemoradiotherapy were included in this study. Patients who had early glottic cancer, nasopharyngeal cancer, distant metastasis, previous RT, or previous definitive surgery were excluded. This study protocol was approved by our institutional review board. The tumor location was hypopharyngeal in 10, oropharyngeal in 6, and laryngeal in 4 patients. Histologically, all lesions were squamous cell carcinoma.
All patients were treated with intensity-modulated RT with a simultaneous integrated boost technique. All of the primary and nodal gross tumor volume, with a 10-mm margin, was defined as the clinical target volume 1 (CTV1); CTV2 included high-risk nodal regions and a 5-mm margin additional to the CTV1; CTV3 included the low-risk nodal volume. A radiation dose of 70 Gy in 35 fractions (2 Gy/fraction) was prescribed to CTV1, 63 Gy in 35 fractions (1.8 Gy/fraction) was prescribed to CTV2, and 56 Gy in 35 fractions (1.6 Gy/fraction) was prescribed to CTV3.
Seventeen of 20 patients (85%) received concurrent chemotherapy. Fifteen patients received concurrent weekly cisplatin chemotherapy (cisplatin 35 mg/m2 intravenous [IV], weekly) and two patients received concurrent cetuximab (cetuximab 400 mg/m2 IV on 7 days before the start of RT, and 250 mg/m2 IV weekly during RT). Two patients with stage T2N0 and T3N0 hypopharyngeal cancer and one patient with clinically node-negative subglottic cancer did not receive concurrent chemotherapy.
Five patients received three cycles of neoadjuvant chemotherapy before CCRT, four of whom were treated with a combined regimen of docetaxel (70 mg/m2 IV on D1), cisplatin (40 mg/m2 IV on D2, D3), and 5-fluorouracil (400 mg/m2 IV on D1–D3) every 3 weeks. The remaining patient was treated with paclitaxel (230 mg/m2 IV on D1) and cisplatin (60 mg/m2 IV on D1) every 3 weeks.
The clinical response of the tumor to RT was evaluated on CT performed 1 month after completion of RT. At 3 months after RT, follow-up CT and PET/CT were performed and salvage surgical treatments were considered for any residual or recurrent locoregional lesions. Three patients with suspected residual lymph nodes underwent salvage neck dissection at 3, 5, and 9 months after RT, respectively. One of them didn’t have any metastatic lymph node at surgical specimen and the others were successfully salvaged. Another three patients received salvage total laryngectomy with or without neck dissection at 3 months, 3 months and 4 months after RT, respectively. Two of them didn’t have any evidence of disease after surgery but the other one patient expired at 3 months after surgery because of the bleeding from common carotid artery pseudoaneurysm.
2. FDG PET/CT imaging and measurement of PET/CT parameters
FDG PET/CT images were acquired in all 20 patients, using a Gemini TF scanner (Philips Healthcare, Cleveland, OH, USA) before and during RT. The timing of scanning during RT ranged from the 3rd to 4th week of RT (median, 26 days from the start of RT). The PET/CT scanning methods used were as previously described [14]. The acquired PET/CT images were transferred to a dedicated workstation and analyzed using the vendor-provided software (The Extended Brilliance Workspace with Fusion Viewer, Philips Healthcare). The software of the workstation provided automatically delineated volume-of-interest (VOI) over the tumor using a threshold of 50% of maximum standardized uptake value (SUVmax) [15]. Metabolic tumor volume (MTV) was defined as those voxels having an SUV greater than 50% of the SUVmax. Total lesion glycolysis (TLG) was calculated by multiplying MTV by the mean standardized uptake value (SUVmean). SUV, MTV, and TLG were measured at the primary tumor site. There were 3 patients whose metabolic parameters were unmeasurable at the primary tumor site after neoadjuvant chemotherapy, and their PET/CT parameters were measured at the metastatic nodal sites. If the tumor could not be distinguished from the background, MTV was set as a single voxel with a volume of 0.1 cm3. SUV was assigned with a default value of 1.0, which was the minimum value [16].
3. Statistical analyses
Receiver operating characteristic (ROC) curve analysis was used to identify the optimal cut-off value for continuous PET/CT parameters, as the maximal point of the sum of sensitivity and specificity. All patients were divided into two subgroups for good or poor outcome in terms of OS or progression-free survival (PFS) for each clinical variable and PET/CT parameter. OS was defined as the time interval between the date of any first treatment and the date of death or last follow-up. PFS was defined as the time from the date of any first treatment to the date of locoregional or distant recurrence. Death without documented recurrence was censored at the time of death. The Kaplan–Meier method was used to calculate the 3-year OS and PFS rates. In univariate analysis, the log-rank test was used to compare the clinical variables and PET/CT parameters. In multivariate analysis, the Cox proportional hazard model was used to identify independent prognostic factors of OS and PFS. Paired t-test was used to compare the PET/CT parameters before and during RT. Statistical significance was defined as a p-value <0.05 (two-sided). All statistical analyses were performed using SPSS version 20.0 software (IBM Corporation, Armonk, NY, USA).
Results
1. Clinical characteristics and FDG PET/CT parameters
The clinical characteristics of the patients are summarized in Table 1. There were 4 (20%) patients with radiological T1 or T2 tumors and 16 (80%) with T3 or T4. Nine patients (45%) were clinical N0 stage, and 11 (55%) were N1 or N2 stage. The clinical response of the tumor to RT was evaluated on neck CT, 1 month after the completion of RT. According to the revised RECIST criteria (v.1.1), 4 patients (20%) showed a complete response (CR), 11 patients (55%) had a partial response (PR), and 5 (25%) had stable disease (SD). In the FDG PET/CT before RT, the SUVmax of the primary tumors ranged from 2.0 to 15.8 g/mL, with a mean value of 6.9 ± 3.8 g/mL. During RT, the SUVmax significantly decreased and ranged between 1.4 and 10.2 g/mL, with a mean value of 4.2 ± 2.5 g/mL. The mean value of SUVmean before RT was 4.7 ± 2.6 g/mL (range, 1.0 to 10.5 g/mL), and that during RT also significantly decreased and was 2.7 ± 1.8 g/mL (range, 1.0 to 7.0 g/mL). Table 2 shows the FDG PET/CT parameters before and during RT.
2. Survival analysis
The median follow-up time was 49 months (range, 42 to 60 months). Fifteen patients (75%) were alive at last follow-up. The 3-year OS rate of all 20 patients was 80.0% and the 3-year PFS was 60.0%.
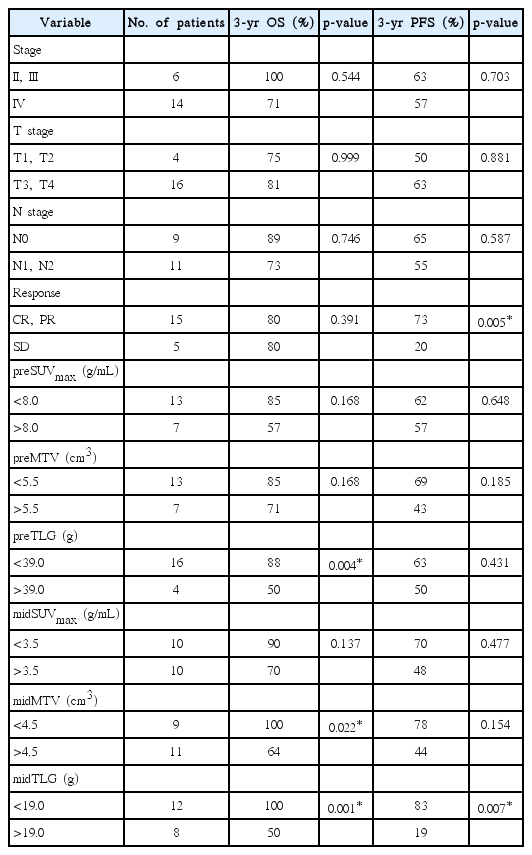
Before RT, the optimal cut-offs for PET/CT parameters derived from the ROC curves were: SUVmax (preSUVmax) = 8.0 g/mL (area under the ROC curve [AUC] = 0.57, p = 0.631), MTV (preMTV) = 5.5 cm3 (AUC = 0.71, p = 0.176), and TLG (preTLG) = 39 g (AUC = 0.68, p = 0.239). The 3-year OS rate was higher in patients with preTLG <39 g than in those with preTLG >39 g (88% vs. 50%; p = 0.004).
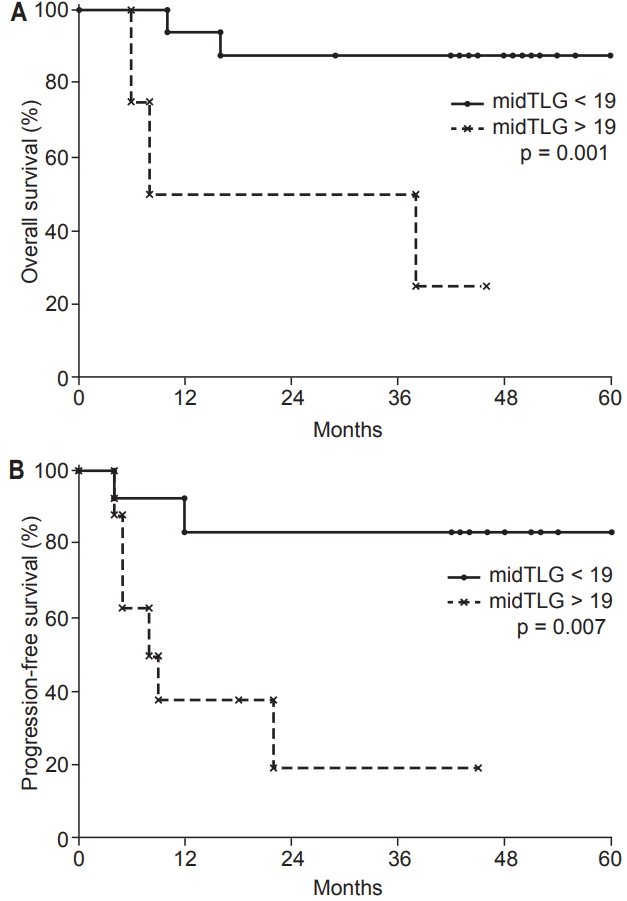
During RT, the optimal cut-offs for PET/CT parameters were: SUVmax (midSUVmax) = 3.5 g/mL (AUC = 0.74, p = 0.116), MTV (midMTV) = 4.5 cm3 (AUC = 0.81, p = 0.040), and TLG (midTLG) = 19.0 g (AUC = 0.91, p = 0.008). The 3-year OS rate was higher in patients with midTLG <19.0 g than in those with midTLG >19.0 g (100% vs. 50%; p = 0.001). OS rate was lower in patients with a higher midMTV (>4.5 cm3) than in those with a lower midMTV (<4.5 cm3) (3-year rate, 64% vs. 100%; p = 0.022).
Patients with tumor response better than PR after RT had a higher PFS rate than those without tumor response to RT (SD) (3-year, 73% vs. 20%, respectively; p = 0.005). The 3-year PFS rate of patients with midTLG >19.0 g was 19% and the rate in patients with midTLG <19.0 g was 83% (p = 0.007). Table 3 shows the 3-year OS and PFS rates according to the clinical variables and metabolic parameters. Multivariate analysis revealed that midTLG as a continuous variable and no tumor response to RT were independent predictors of shorter PFS (p < 0.05) (Table 4). MidTLG was also an independent prognostic factor for OS (p < 0.05) (Table 4). OS and PFS curves according to midTLG are illustrated in Fig. 1.
Discussion and Conclusion
Fractionated RT with concurrent chemotherapy is the standard treatment for locally advanced head and neck cancer, but it takes 6–7 weeks to complete treatment and most patients suffer from acute toxicities. If long-term treatment outcomes could be predicted during RT, the treatment plan for each individual patient could be modified. Therefore, the predictive or prognostic value of interim FDG PET/CT is currently being investigated.
FDG PET/CT is widely used for diagnosis and treatment planning in patients with head and neck cancer; however, the prognostic value of the FDG PET/CT parameters remains under investigation. MTV and TLG are volume-based metabolic parameters derived from VOI-based automated assessments. Because TLG is the product of MTV and SUVmean, the TLG value is a measure of both the volumetric burden and the metabolic activity of a tumor.
MTV or TLG value can be affected by the threshold of VOI. VOI was delineated with variable methods in previous studies that evaluated the prognostic value of volumetric parameters of FDG PET/CT. A fixed value of SUV or percentage of SUVmax is used for VOI determination. A fixed SUV of 2.0–3.0 and 40%–50% of SUVmax were frequently used thresholds in previous studies [17] and 50% of SUVmax was used as a threshold in the present study because it has been identified as a reasonable choice in phantom studies [15].
TLG was a significant prognostic factor for OS in patients with tonsil cancer [18]. In a study of 74 oropharyngeal cancer patients who received CCRT, both pre-treatment and midtreatment MTV were associated with OS and PFS [19]. In a meta-analysis of more than 1,100 head and neck cancer patients, both MTV and TLG of pretreatment PET/CT were prognostic factors for OS [17]. The cut-off values of studies included in that meta-analysis were 7.7–45 cm3 for MTV and 55–330 g for TLG. In the current study, which had a relatively small number of head and neck cancer patients, a higher TLG or MTV before RT was associated with lower OS rates. The cut-off values of our study (5.5 cm3 for MTV and 39.0 g for TLG) were lower than other studies. This is probably because one-fourth of our patients underwent neoadjuvant chemotherapy before FDG PET/CT.
Min et al. [20] reported that among the parameters of SUVmax, MTV, and TLG of PET/CT during RT, TLG was the best prognostic indicator of oncologic outcome. Also in our study, TLG during RT was the most statistically significant prognostic factor for OS and PFS. However, although the results of the two studies were similar, they used different TLG cut-off values (9.4 vs. 19.0). Differences in the study populations, timing of scanning during RT, PET/CT scanner used, image analysis software, and methods for obtaining MTV and TLG may have caused the different optimal cut-off values. Volume-based metabolic parameters such as MTV and TLG were changed time-dependently [21,22]. When early scanning at 60 minutes after FDG injection was compared with late scanning at 120 minutes, TLG was significantly increased at delayed phase.
Chen et al. [23] measured the SUVmax at both the primary tumor and metastatic lymph node before and during RT. The authors demonstrated that a higher interim SUVmax or lower reduction ratio of the SUVmax at the primary tumor was a poor prognostic factor (they did not analyze MTV or TLG). Other investigators have suggested that measurements of metabolic parameters on PET/CT during RT were more prognostic at nodal sites than in the primary tumor, because they found less variability in measurements at the nodal sites [24]. Lin et al. [25] identified nodal SUVmean and a reduction of nodal MTV and TLG of >50% during RT as prognostic factors. Which one has more significant value for predicting treatment outcomes, metabolic parameters measured at primary tumor or nodal site, remains a research issue.
This study has several limitations. The small number of patients and heterogeneity of primary sites, stages of disease, and treatment methods might have resulted in unreliable cut-off value of PET/CT parameters. Previous other studies also had limitation of small sample size and heterogeneity of the population [26].
The results of our study and of these previous studies suggest that FDG PET/CT during CCRT or RT could be used to assess treatment outcomes. We acquired FDG PET/CT images in the third to fourth week of RT, but earlier assessments (i.e., 2 weeks after the start of RT) are thought to be more favorable [26]. Performing FDG PET/CT early during RT could be useful in differentiating metabolic changes from inflammatory changes and in enabling early decisions to be made regarding modification of treatment plans.
There remain many unsolved issues regarding the optimal timing of FDG PET/CT during RT, the selection of the most useful PET/CT parameters and their optimal cut-offs, and choice of the most appropriate treatment plans for patients identified as having poor prognostic factors. Thus, larger scale investigations with more a homogeneous patient group are needed.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. NRF-20161A2B4012095).