 |
 |
AbstractPurposeWe evaluated the prognostic factors and clinical outcomes of 56 patients with vulvar cancer treated with curative radiotherapy (RT) or concurrent chemoradiotherapy.
Materials and MethodsOverall survival (OS) and disease-free survival (DFS) were assessed retrospectively. Prognostic factors evaluated included age, International Federation of Gynecology and Obstetrics (FIGO) stage, TNM classification, tumor size, treatment modality, RT duration, and RT field. The association between the tumor human papillomavirus (HPV) status and survival was analyzed in 35 patients.
ResultsDuring the median follow-up of 2.8 years (range, 0.3 to 18.9 years), 21 patients (37.5%) experienced treatment failure. Fifteen patients (27%) had local failure: nine (16%) local failure only, three (5%) locoregional failure, two (4%) local and distant failure, and one (2%) locoregional and distant failure. Of 56 patients, seven (13%) had persistent disease at the first follow-up at 2 months and all but one died within a year after completing RT. The 5-year OS and DFS were 51.6% and 44.0%, respectively. In multivariate analysis, clinical size ≥3 cm predicted a poor prognostic factor for DFS (p = 0.040) and age (≥70 years) was poor prognostic for DFS (p = 0.032) and OS (p = 0.048). Patients with HPV-positive tumors tended to have better 5-year OS and DFS, but the differences were not significant statistically.
IntroductionVulvar cancer is a relatively uncommon disease, accounting for about 5% of all gynecological malignancies [1]. According to United States' statistics from the Surveillance, Epidemiology, and End Results registries, nearly 90% of patients with vulvar cancer are diagnosed at an early stage, whereas advanced disease predominantly occurs in older patients, with poor treatment outcomes [2]. Traditionally, surgical management has played a major role in the curative treatment for vulvar cancer, and radiotherapy (RT) has been used as an adjuvant therapy or in the palliative context. Disease control of the inguinofemoral region is regarded as an important approach to the treatment of vulvar cancer, based on the report of the Gynecologic Oncology Group (GOG), which assigns an extremely important prognostic value to groin lymph node (LN) metastasis [3]. Although radical vulvectomy with inguinofemoral lymphadenectomy with or without pelvic nodal area, produces favorable oncological outcomes as groin recurrence rates of <10% [456], it leaves the patient with significant postoperative morbidity, including psychological problems, wound disruption, lymphedema, or sexual dysfunction [789]. A high mortality rate (up to 20%) has been reported in cases of advanced-stage disease following surgical procedures [10]. The survival outcomes after definitive RT with chemotherapy vary from 46.2% to 65.0% [111213]. Although the probability of tumor control by RT in patients with small tumors is similar to that for surgery, the results have generally been poor in terms of both the local control rate and the treatment-related toxicity in patients with stages III-IV vulvar cancer [14]. In recent phase II GOG studies of concurrent chemoradiotherapy (CCRT), clinical complete remission was achieved in 47.9% to 63.8% of patients and pathologic complete remission in 31.0% to 50.0% [1516]. However, in many cases, patients require tumor resection because the tumor has a less-than-complete response to CCRT, and complication rates are higher and morbidity significantly greater in those cases [17]. Given the rarity of the disease, few randomized trials have compared the efficacy of surgery with RT as the definitive tools for its treatment. With this background, we evaluated the clinical outcomes and prognostic factors of patients with vulvar cancer treated primarily with RT. The study was a retrospective review of the medical records of 7 institutions in Korea.
Materials and MethodsThe records of 118 patients with carcinoma of the vulva, who were treated at 7 institutions in Korea between 1998 and 2011, were retrospectively evaluated. Patients were included if they had biopsy-proven squamous cell carcinoma or adenocarcinoma of the vulva and had received RT or CCRT with curative intent. Patients were excluded from the analysis if they had as follows: 1) histological evidence of melanoma, basal cell carcinoma, or adenoid cystic carcinoma; 2) distant metastasis, other than pelvic LN metastasis; 3) had received neoadjuvant chemotherapy; or 4) had undergone radical vulvectomy. Ultimately, 56 patients were included in the analysis.
1. Patient demographicsThe patients' demographic data are shown in Table 1. The median age was 71 years (range, 28 to 90 years) and the majority of patients (98%) had squamous cell carcinoma. All patients were staged with the surgical pathological staging system for vulvar cancer of the International Federation of Gynecology and Obstetrics (FIGO), established in 2009. Fourteen patients (25%) had a clinical T3 classification and 26 (36%) had tumors ≥3 cm. Of the 56 patients analyzed, 32 (57%) had inguinal LN metastasis and 10 (18%) had pelvic LN metastasis. Eight patients (14%) had FIGO stage I disease, eight (14%) stage II, 20 (36%) stage III, and 20 (36%) stage IV.
2. Pretreatment evaluation and treatment modalitiesAll patients underwent physical and pelvic examinations, routine laboratory testing, chest radiography, colposcopy, cystoscopy, and sigmoidoscopy. When advanced-stage disease was diagnosed, further evaluation with magnetic resonance imaging (MRI) or positron emission tomography-computed tomography was performed.
Of all the patients, 23 (41%) received CCRT and 33 (59%) only RT (Table 2). The selection of the treatment modality was at the discretion of the treating physicians. The general schema for CCRT involved weekly cisplatin or cisplatin plus 5-fluorouracil concurrently with external-beam radiation. The radiation fraction size was generally 1.8 to 2 Gy, delivered once a day, with a median total dose of 65 Gy (range, 45 to 130 Gy). The radiation technique and dose were individualized for each patient, depending on the site and volume of the tumor. The majority of patients (91%) received two-dimensional RT (2D-RT) or three-dimensional conformal RT (3D-CRT). Until the late 1990s 2D-RT was predominantly used and 3D-CRT was used from early 2000. In terms of the extent of the RT field, 42 patients (75%) received RT to the primary vulva, both inguinal LNs, and the whole pelvis. A boost to the primary vulvar site with brachytherapy (n = 8) or external-beam RT (n = 44) was applied in 52 (93%) patients. The median overall treatment time was 57 days (range, 36 to 111 days).
3. Human papillomavirus (HPV) statusGenomic deoxyribonucleic acid (DNA) was extracted from the paraffin-embedded tissue sections or frozen tissues using the QIAamp DNA mini kit (Digene Corp., Gaithersburg, MD, USA). When the cell samples were obtained by scraping the vulvar lesions, the brush containing the cellular material was placed directly in a vial containing PreservCyt solution (Digene Corp.) before testing. The HPV DNA titer was measured with a commercially available second-generation hybrid capture microplate-based HPV DNA test (HC2 test, Digene Corp.). The HC2 test uses an RNA probe mixture of 13 high-risk HPV types (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). The relative light unit (RLU)/cutoff ratios were calculated as the ratio of the specimen luminescence to the luminescence of the 1.0 pg/mL HPV 16 cutoff standard. RLU/cutoff ≥1.0 was considered positive, according to the manufacturer's recommendation (Digene reference no. 5199-1220).
4. StatisticsAcute toxicity, measured from the initiation of treatment to three months after RT or CCRT, was assessed with the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0) [18], from the medical records of the patients at each institution. Late toxicity was graded according to the Radiation Therapy Oncology Group Late Morbidity Scoring Criteria [19]. A complete response was defined as the complete clinical or radiological disappearance of gross disease, based on a physical examination and an MRI scan, which were performed three months after the completion of treatment. Local failure was defined as either persistent disease at three months after the completion of treatment or any local recurrence. Regional failure was defined as either persistent groin (inguinofemoral) disease at three months after the completion of treatment or any groin nodal recurrence. Distant failure was defined as the development of any distant disease including pelvic nodal failure irrespective of RT field. Disease-free survival (DFS) was calculated from the start of treatment to the date of locoregional or distant failure or death from any cause. Any recurrence or relapse, or death from any cause, was regarded as an event. Overall survival (OS) was calculated from the start of treatment to the date of death or the last follow-up. The 5-year actuarial rates of OS and DFS were analyzed by Kaplan-Meier method. Univariate analyses of prognostic factors for OS and DFS were performed with the log-rank test. Factors independently associated with OS or DFS were identified with a multivariate analysis using the Cox proportional hazards regression model. The p-values of less than 0.05 were considered significant. All statistical analyses were performed with SPSS ver. 19.0 (IBM, Armonk, NY, USA).
ResultsThe median follow-up period was 2.8 years (range, 0.3 to 18.9 years) for all patients. Of the 56 patients, 29 (51.8%) were alive at follow-up, 15 (26.8%) had died from vulvar cancer itself or treatment-related complications, and 11 (19.6%) had died from age-related or other causes. One patient was lost to follow-up after the first follow-up schedule.
1. Patterns of treatment failureIn total, 21 patients (37.5%) experienced treatment failure (Fig. 1). Local failure was observed in 15 patients (27%): 9 (16%) with local failure only, 3 (5%) with locoregional failure, 2 (4%) with local and distant failure, and 1 (2%) with locoregional and distant failure. Of 56 patients, 7 (13%) showed persistent disease at the first follow-up at 2 months and all but one died within a year after completing RT. Distant failure was identified in 9 patients (16%): 4 patients (7%) had distant failure only (2 lung, 1 pelvic failure outside the RT field, 1 unknown due to incomplete data), 5 (9%) had distant failure with either local or regional failure or both of them (1 lung, 1 pelvic bone, 2 para-aortic LN, and 1 pelvic failure outside the RT field). No patient had regional failure only.
2. Survival and prognostic factorsThe 5-year OS and DFS rates were 51.6% and 44.0%, respectively (Fig. 2). In a univariate analysis (Table 3), age was the strongest prognostic factor for OS (p = 0.009) and DFS (p = 0.008). Neither the FIGO stage, inguinal LN metastasis, nor pelvic LN metastasis was a prognostic factor for OS or DFS, whereas tumor size ≥3 cm was a prognostic factor for OS (p = 0.043) and DFS (p = 0.019). A radiation dose of ≥65 Gy to the gross tumor volume appeared to be associated with a higher failure rate (Table 3). Since it was interpreted that this finding was resulted from the use of a higher radiation dose in patients whose interim response was poor, a radiation dose of ≥65 Gy was not included in multivariate analysis. When age, size of the primary lesion, inguinal LN metastasis, pelvic LN metastasis, and treatment modality were considered in the multivariate analysis, the only prognostic factor for OS and DFS was age (p-value, 0.048 and 0.032, respectively), whereas tumor size ≥3 cm was a poor prognostic factor for DFS only (p = 0.040) (Table 4).
3. Treatment-related toxicityThe most common acute toxicity affected the skin (Table 5). Grade 3 acute skin toxicity was observed in 12 patients (21%). Seven patients (13%) experienced grade 2 acute gastrointestinal toxicity, which was transient and controlled with supportive care, and one patient suffered grade 3 acute gastrointestinal toxicity. Grade 2 acute genitourinary toxicity was observed in 10 patients (18%). Only one patient suffered grade 3 acute hematological toxicity.
4. Subanalysis of tumor HPV status and survivalHPV-positive vulvar cancer was more common in younger patients (median age was 64.5 years in HPV-positive patients and 75 years in HPV-negative patients). Nine (60%) of the 15 HPV-positive patients were <70 years old (Pearson χ2, p = 0.036). The HPV-positive patients tended to have better 5-year OS and DFS, but these trends were not significant in the univariate analysis (all p > 0.05) (Fig. 3).
Discussion and ConclusionBecause the disease is rare and surgery is the mainstay treatment for early vulvar cancer, limited data are available on the outcomes of primary RT for vulvar cancer. Many published studies have included the results for heterogeneous groups of patients, with disease severity extending from FIGO stage I to stage IV, so the treatment outcomes reported tend to vary. Tans et al. [13] suggested that the 4-year actuarial rates for locoregional control and OS were 75% and 65%, respectively, in locally advanced vulvar carcinoma. In a comparison of primary surgery and chemoradiation for FIGO stage III-IV vulvar cancer, patients treated with definitive chemoradiation showed an OS rate of 75% [14]. In contrast, Han et al. [11] reported 5-year rates for relapse-free survival and OS of 43% and 54%, respectively, in 14 patients who received primary chemoradiation therapy. Lee et al. [12] reported 5-year DFS and OS rates of 42.2% and 46.2%, respectively, in heterogeneous patients treated with primary or adjuvant RT with or without concomitant chemotherapy.
Our study shows that the general treatment outcomes of vulvar cancer are poor, with 5-year OS, and DFS rates of 51.6% and 44.0%, respectively. The main cause of treatment failure was local failure, and a substantial amount of persistent local disease was noted at the end of RT in 13% (7/56) of patients. One reason for the considerably higher local failure rate was the RT technique used. These patients were treated with an RT technique that evolved from 2D-RT to intensity-modulated RT (IMRT) between 1989 and 2012. It is difficult to deliver a homogeneous radiation dose to the target area, which includes the vulvar, the inguinofemoral LNs, and even the pelvic LNs, with 2D-RT or 3D-CRT. Therefore, techniques such as IMRT, which can deliver different doses to the primary site and the regional LNs, are required. According to the report of Beriwal et al. [20], the best dose distribution for the treatment of vulvar carcinoma can be achieved with IMRT. They reported favorable clinical outcomes, with complete clinical responses, in 74% of patients, complete pathological responses in 64%, and a 2-year disease-specific survival rate of 75%, with no grade 3 acute or late morbidity when preoperative CCRT was performed with IMRT in patients with FIGO stage II-IVA vulvar cancer [21].
It has been observed that there are two different etiologic pathogenesis in vulvar carcinogenesis [2223]. The first is often observed in younger patients less than 50 years old who more frequently smoke and are at higher risk for HPV infection and whose invasive cancers are commonly associated with vulvar intraepithelial neoplasia (VIN). The second is seen in elderly patients whose disease may be unrelated to HPV infection or VIN but lichen sclerosis and chronic inflammation are common. The present study also showed that HPV-positive vulvar cancer was more common in younger patients (median age was 64.5 years in HPV-positive patients and 75 years in HPV-negative patients) although it was not in less than 50 years old. It has not been clearly investigated for correlation between HPV status and survival outcome in vulvar cancer. Alonso et al. [24] reported that no differences were seen between HPV positive and HPV negative tumors in terms of 5-year DFS (39.8% vs. 49.8%, p = 0.831) and OS (67.2% vs. 71.4%, p = 0.791). Pinto et al. [25] also suggested that high-risk HPV DNA was not associated with prognostic significance for death (hazard ratio, 0.9) and recurrence (hazard ratio, 0.8) in invasive vulvar carcinoma following radical vulvectomy. We also investigated correlation between HPV status and survival outcome but no statistical difference was shown for OS, DFS according to HPV status. In this study, however, the information on HPV status was limited to a subset of patients and differences in the prognoses of patients with different etiologies (HPV-related or chronic-inflammation-related carcinogenesis) could not be determined. In the future, more investigation would be necessary for the prognosis according to HPV status.
Compared with the acute skin grade 3 toxicity rate of 21% observed in the current study, IMRT seems to entail a lower risk of skin toxicity, with a good clinical response. In our study, IMRT was used in only five patients (8.9%), two of whom treated with IMRT alone suffered local recurrence, leading to their deaths whereas the remaining 3 patients were treated with CCRT (concomitant platinum-based chemotherapy with IMRT). A previous study showed that chemoradiation achieved a better local control rate and survival rate than RT alone when used as the primary treatment for vulvar cancer [11], so concomitant chemotherapy with RT should be considered to improve the local control rate and perhaps the survival rate.
One of the limitations of our study was that we could not collect accurate data for the tumor response during or at the end of RT. In many cases, the medical records did not determine whether the tumor had responded completely on image/physical examination. A higher radiation dose is associated with a better tumor control, however a radiation dose of ≥65 Gy to the gross tumor volume appeared to be associated with a higher failure rate in this study. We interpret this finding as resulting from the use of a higher radiation dose in patients whose interim response was poor. However, we have to admit that the limitation of this study made it conjecture. In the future, further prospective study would be necessary to investigate the correlation between radiation dose and tumor response. Secondly, we are unable to report any late toxicity in this study because the medical records for many of these patients were unfaithful. Two patients who received over 100 Gy radiation to the primary site could not be assessed for late toxicity because they were lost to follow-up. Considering that insufficient fracture was reported from up to 45% of the patients after pelvic RT in cervical cancer [26], several patients in our study might also suffered from insufficient fracture after treatment.
Our data do not show that the survival rates differed according to the clinical stage of the disease, but only age and tumor size most effectively predicted the survival outcomes. We retrospectively investigated the clinical outcomes and prognostic factors of patients with vulvar cancer treated primarily with RT. The median age was 71 years (range, 28 to 90 years). Of sixteen patients with stage I and II disease, 9 (56%) were older than 70 years. In general, medically inoperable patients with early stage disease might be referred to receive RT, consequently, which had resulted in poor outcome in those patients. A substantial amount of persistent local disease was also noted at the end of RT in 13% (7/56) of patients, who were all older than 70 years. It could explain why surgical consolidation therapy was not considered in those patients. In the future, further investigation would be necessary to determine which could be the treatment of choice for old age group.
In conclusion, this study suggests that a tumor size ≥3 cm is associated with a negative clinical outcome in DFS in patients with treated with curative RT. However, age ≥70 years indicates the most significant poor prognosis for OS and DFS. In the future, further study would be necessary to determine which treatment should be considered for old age group and more investigations are needed to define the prognoses of old age group with different etiologies according to HPV-related or chronic-inflammation-related carcinogenesis.
AcknowledgmentsThis study was supported by a grant from the National Cancer Center, Korea (No. 1310070 and 1310300).
References1. American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012.
2. Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 2008;108:577–583, PMID: 18155274.
3. Homesley HD, Bundy BN, Sedlis A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol 1991;164:997–1003, PMID: 2014852.
4. Bell JG, Lea JS, Reid GC. Complete groin lymphadenectomy with preservation of the fascia lata in the treatment of vulvar carcinoma. Gynecol Oncol 2000;77:314–318, PMID: 10785485.
5. De Hullu JA, Hollema H, Lolkema S, et al. Vulvar carcinoma: the price of less radical surgery. Cancer 2002;95:2331–2338, PMID: 12436439.
6. Kirby TO, Rocconi RP, Numnum TM, et al. Outcomes of Stage I/II vulvar cancer patients after negative superficial inguinal lymphadenectomy. Gynecol Oncol 2005;98:309–312, PMID: 15975642.
7. Andersen BL, Hacker NF. Psychosexual adjustment after vulvar surgery. Obstet Gynecol 1983;62:457–462, PMID: 6888823.
8. Gould N, Kamelle S, Tillmanns T, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329–332, PMID: 11531288.
9. Lin JY, DuBeshter B, Angel C, Dvoretsky PM. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol 1992;47:80–86, PMID: 1427407.
10. Hoffman MS. Squamous-cell carcinoma of the vulva: locally advanced disease. Best Pract Res Clin Obstet Gynaecol 2003;17:635–647, PMID: 12965136.
11. Han SC, Kim DH, Higgins SA, Carcangiu ML, Kacinski BM. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiat Oncol Biol Phys 2000;47:1235–1244, PMID: 10889377.
12. Lee J, Kim SH, Kim G, et al. Treatment outcome in patients with vulvar cancer: comparison of concurrent radiotherapy to postoperative radiotherapy. Radiat Oncol J 2012;30:20–26, PMID: 23120740.
13. Tans L, Ansink AC, van Rooij PH, Kleijnen C, Mens JW. The role of chemo-radiotherapy in the management of locally advanced carcinoma of the vulva: single institutional experience and review of literature. Am J Clin Oncol 2011;34:22–26, PMID: 20087157.
14. Landrum LM, Skaggs V, Gould N, Walker JL, McMeekin DS. Comparison of outcome measures in patients with advanced squamous cell carcinoma of the vulva treated with surgery or primary chemoradiation. Gynecol Oncol 2008;108:584–590, PMID: 18155755.
15. Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol 2012;124:529–533, PMID: 22079361.
16. Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 1998;42:79–85, PMID: 9747823.
17. Woelber L, Trillsch F, Kock L, et al. Management of patients with vulvar cancer: a perspective review according to tumour stage. Ther Adv Med Oncol 2013;5:183–192, PMID: 23634196.
18. National Cancer Institute (US). Common terminology criteria for adverse events (CTCAE) version 4.0. Bethesda, MD: National Institutes of Health; National Cancer Institute; 2009.
19. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–1346, PMID: 7713792.
20. Beriwal S, Heron DE, Kim H, et al. Intensity-modulated radiotherapy for the treatment of vulvar carcinoma: a comparative dosimetric study with early clinical outcome. Int J Radiat Oncol Biol Phys 2006;64:1395–1400, PMID: 16442238.
21. Beriwal S, Coon D, Heron DE, et al. Preoperative intensitymodulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol 2008;109:291–295, PMID: 18455637.
23. Madeleine MM, Daling JR, Carter JJ, et al. Cofactors with human papillomavirus in a population-based study of vulvar cancer. J Natl Cancer Inst 1997;89:1516–1523, PMID: 9337348.
24. Alonso I, Fuste V, del Pino M, et al. Does human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma? Gynecol Oncol 2011;122:509–514, PMID: 21652058.
25. Pinto AP, Schlecht NF, Pintos J, et al. Prognostic significance of lymph node variables and human papillomavirus DNA in invasive vulvar carcinoma. Gynecol Oncol 2004;92:856–865, PMID: 14984953.
26. Oh D, Huh SJ. Insufficiency fracture after radiation therapy. Radiat Oncol J 2014;32:213–220, PMID: 25568849.
Fig. 3Survival rates according to human papillomavirus (HPV) status: (A) overall survival (OS) and (B) disease-free survival (DFS).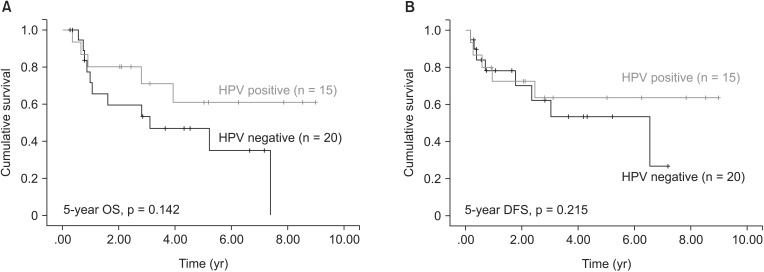
Table 3Univariate analysis of prognostic factors for overall and disease-free survival (n = 56)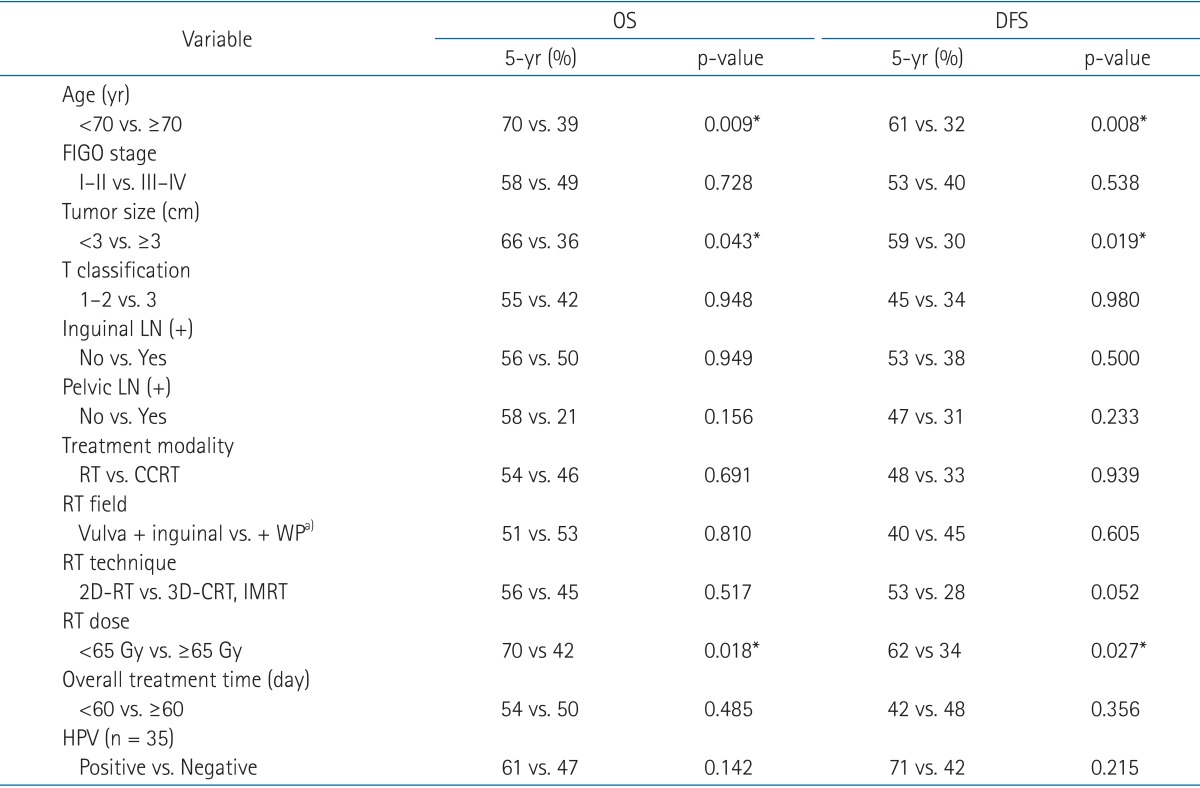 OS, overall survival; DFS, disease-free survival; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; 2D-RT, two-dimensional radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; HPV, human papillomavirus. a)+ WP = vulva + inguinal + whole pelvis. *Statistically significant at p < 0.05. |
|
||||||||||||||||||||||||||||||||||||||||

 |
 |