 |
 |
AbstractPurposeWe compared how doses delivered via two-dimensional (2D) intracavitary brachytherapy (ICBT) and three-dimensional (3D) ICBT varied anatomically.
Materials and MethodsA total of 50 patients who received 30 Gy of 3D ICBT after external radiotherapy (RT) were enrolled. We compared the doses of the actual 3D and 2D ICBT plans among patients grouped according to six anatomical variations: differences in a small-bowel V2Gy, small bowel circumference, the direction of bladder distension, bladder volume, sigmoid V3.5Gy, and sigmoid circumference. Seven dose parameters were measured in line with the EMBRACE recommendations.
ResultsIn terms of bladder volume, the bladder and small-bowel D2cc values were lower in the 150–250 mL bladder volume subgroup; and the rectum, sigmoid, and bladder D2mL values were all lower in the >250 mL subgroup, for 3D vs. 2D ICBT. In the sigmoid V3.5Gy >2 mL subgroup, the sigmoid and bladder D2mL values were significantly lower for 3D than 2D ICBT. The bladder D2mL value was also significantly lower for 3D ICBT, as reflected by the sigmoid circumference. In patients with a small bowel V2.0Gy >10 mL or small bowel circumference >15%, most dose parameters were significantly lower for 3D than 2D ICBT. The bladder distension direction did not significantly affect the doses.
IntroductionThe National Comprehensive Cancer Network Guidelines indicate that intracavitary brachytherapy (ICBT) is an essential form of definitive radiotherapy (RT) for cervical cancer [1]. Earlier two-dimensional (2D) ICBT methods were well defined in the International Commission on Radiation Units (ICRU) Report 38 [2]. In the 2000s, developments in treatment planning and imaging techniques led to the introduction of computed tomography (CT)-based and magnetic resonance imaging (MRI)-based three-dimensional (3D) ICBT. The 3D ICBT guidelines of the Groupe Européen de Curiethérapie–European Society for Radiotherapy and Oncology (GEC-ESTRO) Working Group are now widely used [3-6].
Previous studies found significant differences in the dose distribution profiles of 3D ICBT and 2D ICBT [7], affecting the incidences of complications in healthy organs such as the bladder and rectum [8,9]. Another study found that interfractional differences in dose distributions depended on variations in the locations of healthy organs [10], suggesting that organ location may affect dose distribution. In recent studies evaluating clinical outcomes in terms of local control and toxicities, delivery of a prescribed dose to the high-risk clinical target volume (HR-CTV) via 3D ICBT improved local control and reduced gastrointestinal and genitourinary toxicities such as rectal bleeding and cystitis [11-15].
We implemented MRI-based treatment when 3D ICBT was introduced in our institution. We found that the brachytherapy doses prescribed by treatment plans were affected by individual anatomical variations. Despite plan optimization, the target dose and the tolerance doses of organs at risk (OARs) were unsatisfactory in some treatment plans. However, few institutions engage in 3D ICBT; most employ 2D ICBT. Therefore, we compared the dose distributions of 2D and 3D ICBT by anatomical variations.
Materials and Methods1. Patient characteristicsFrom May 2015 to December 2016, we studied 50 patients who underwent RT to treat histologically confirmed cervical cancer in a single institute. All patients received external RT (median dose, 45 Gy; range, 36 to 54 Gy, 1.8 Gy/fraction, 5 fractions/week) and MRI-based 3D ICBT (30 Gy; 5 Gy/fraction, 2 fractions/week). The median age was 53.5 years (range, 33 to 87 years). Most patients had stage IIB squamous cell carcinoma, and almost all underwent concurrent chemoradiotherapy (CCRT). Brachytherapy generally employed a 30° tandem with ring-type ovoid. Patient characteristics are listed in Table 1.
2. 3D ICBTAt our institution, ICBT is a component of definitive CCRT or RT for cervical cancer. In general, ICBT is performed after delivery of external RT of about 45 Gy; ICBT simulation is performed when the external RT attains 40 Gy. Prior to treatment, the patient is given an enema to control bladder and rectal volumes, and intravenous analgesia is used to prevent pain. The patient is taken to the brachytherapy room and placed in a lithotomy positioning device. A Foley catheter is introduced into the bladder and approximately 150 mL normal saline injected, due to reduce unwanted anatomical variations. Tandem and ovoid applicators are inserted into the uterus/fornix via the vagina, and a rectal retractor then inserted to increase the distance between the rectum and cervix. After applicator installation, CT is performed to check tandem positioning, bladder volume, and small bowel distribution around the treatment site. If these are satisfactory, MRI is performed, and the physician uses the MRI images to contour the target and normal organs. A physicist implements 3D ICBT using the dose distribution of the 2D ICBT plan (point A prescription), and the 3D ICBT plan is optimized to meet the required criteria using the isodose lines and pre-drawn structures. A physician confirms the plan, and twice-weekly treatment follows.
3. Dose distributions by planning methodWe reviewed the MRI-based ICBT plans of all patients. To enable comparison of the 2D and 3D ICBT plans, we created a 2D ICBT plan with the same dose prescriptions to point A as those on the contoured MR images used for treatment planning. Seven structures were contoured on MR images: gross tumor volume (GTV), HR-CTV, intermediate-risk CTV (IR-CTV), the rectum, the bladder, the sigmoid colon, and the small bowel. The target volumes were defined according to the recommendations of the GEC-ESTRO Working Group (I) [3].
We defined six anatomical differences that may affect dose distribution (Fig. 1), as follows: (1) bowel V2Gy (the volume of the bowel receiving a dose >2 Gy at point A in 2D ICBT); (2) bowel circumference (the proportion of the circumference incorporated in the V2Gy region around the uterus); (3) bladder distension direction (the direction in which the bladder extends when filled with urine); (4) bladder volume (the volume of the bladder when filled with urine or normal saline); (5) the sigmoid V3.5Gy (the volume of the sigmoid colon receiving a dose >3.5 Gy at point A in 2D ICBT); and (6) the sigmoid circumference (the proportion of the circumference incorporated in the V3.5Gy region around the uterus). The 2 Gy and 3.5 Gy doses were based on the tolerance dose of each organ, with consideration of the external radiation doses to the whole pelvis. The circumference was the mean circumference ratio of each MRI slice along the length of the tandem. Seven dose parameters were measured in reference to the above anatomical variations: GTV D98%, HR-CTV D98%, IR-CTV D98%, rectum D2mL, sigmoid D2mL, bladder D2mL, and small bowel D2mL (Fig. 2). These parameters are those that the EMBRACE guidelines recommend for consideration when performing 3D ICBT [11]. The D98% is the dose received by 98% of the target volume, and the D2mL the minimum dose received by a 2 mL volume. Dose parameters were converted to equivalent doses in 2 Gy (EQD2) values.
The mean dose parameters were compared between 2D and 3D ICBT using the paired t-test and the Wilcoxon signed-rank test. Differences with p-values <0.05 were considered significant.
ResultsBladder variations were investigated in terms of the direction of bladder distension and bladder volume. The directions of distension were subdivided into superior-inferior, anterior-posterior, and bilateral (6, 36, and 8 women, respectively); bladder volumes were subdivided into ≤150, 150–250, and ≥250 mL. We found no significant differences in any dose parameters of the target volume or healthy organs by the direction of bladder extension. However, each bladder volume subgroup exhibited a significant difference in at least one dose parameter (Table 2). Although none of the target volume dose parameters (except GTV D98% in 150–250 mL subgroup) differed significantly between 2D and 3D ICBT. The rectum D2mL (p = 0.010), sigmoid D2mL (p = 0.046), and bladder D2mL (p = 0.001) were significantly lower with 3D ICBT than 2D ICBT in the bladder volume >250 mL subgroup.
In terms of sigmoid V3.5Gy analysis, the dose distributions of 2D ICBT and 3D ICBT were compared for three patient subgroups with volumes 0, 0–2, and >2 mL. The GTV D98% was smaller for 3D than 2D ICBT, but the other target volume dose parameters did not differ significantly. The sigmoid D2mL (p = 0.042) and bladder D2mL (p = 0.013) differed significantly in the sigmoid V3.5Gy >2 mL subgroup (Table 3).
The sigmoid circumference was divided into three subgroups: 0%, 0%–10%, and >10% (11, 26, and 13 women, respectively). As was true of the sigmoid V3.5Gy, we found no significant differences in any target volume dose parameters except the GTV D98%. The bladder D2mL was significantly lower for 3D ICBT (Supplementary Table S1). Even if the circumference percentage ratio was small, the bladder D2mL was significantly reduced in 3D ICBT.
The small bowel V2.0Gy and circumference were divided into three subgroups: 0, 0–10, and >10 mL (21, 19, and 10 women, respectively) and 0%, 0%–15%, and >15% (21, 21, and 8 women, respectively). In subgroups with small bowel V2.0Gy over 10 mL and circumference ratios over 15%, most of the target dose parameters were significantly reduced for 3D than 2D ICBT. In the subgroup with a small bowel V2.0Gy over 10 mL, both of the bladder and bowel D2mL were significantly decreased. In the subgroup with a bowel circumference ratio over 15%, both of the sigmoid and bowel D2mL values were significantly decreased. The bladder D2mL was not significantly affected (Supplementary Tables S2 and S3).
Discussion and ConclusionWe thought that the most important subgroup in this study was the group with a bladder volume >250 mL. As doses are prescribed in reference to anatomical variations in 3D ICBT, the dose to target may be lower in 3D ICBT than in 2D ICBT. However, in the subgroup mentioned above, the advantage afforded by 3D ICBT is clearest: the radiation dose to normal organs is reduced without compromising the target volume dose. Because the bladder has a higher tolerance dose compared to other organs [11], it has less effect on the target even if it contains a larger volume. Also, It lies directly in front of the uterus, as the bladder volume increases, the small bowel and sigmoid colon can be pushed anteriorly, superiorly, and laterally with respect to the uterus. This can prevent the small bowel and sigmoid colon from exposure to high radiation doses.
Many studies have compared the OAR doses of 2D and 3D ICBT. Pelloski et al. [16] compared doses delivered by 2D ICBT and CT-based ICBT plans to the bladder and rectum of 60 patients treated from 2001 to 2006. The bladder and rectal 2D ICBT reference doses were those of the report of the ICRU. The median bladder volume at the bladder reference dosing point was 13 mL, thus lower than the bladder D2mL dose (mean difference 680 ± 543 cGy; p < 0.001) [16]. Tan et al. [17] performed a similar study comparing the reference point to the organ-2-mL doses in 10 patients. They found significant difference in the bladder dose (per fraction, p = 0.000; total dose, p = 0.004). The cited studies compared the reference point doses of 2D and 3D ICBT, but measured different parameters. Therefore, we evaluated differences in treatment plan doses using the same parameters (Table 4).
Some studies evaluated the movements of internal organs dosimetrically rather than clinically when measuring dose changes associated with intra- and inter-fractional movements [10,18]. Kobayashi et al. [10] measured dose variations between fractions. After delivery of external RT, ICBT was performed four times (total dose, 24 Gy) and CT images obtained after each treatment; organs were contoured on the images and the organ doses associated with each treatment calculated. The differences in the cumulative doses for each index were: bladder D2mL, 0.3±0.8 Gy; bladder D0.1mL, 2.2±0.6 Gy. Mazeron et al. [18] subjected intra-fractional organ movements to dose analyses by performing MRI after procedures featuring ICBT simulation, and CT on days 1–3 of treatment, when pulsed-dose-rate brachytherapy was employed. They found no significant movement of the bladder or sigmoid colon during treatment [18]. Thus, these findings suggest that the internal organs are always in motion, and that organ positions differ among patients. As we delivered high-dose-rate brachytherapy, there was different dose rate delivery concept between this study and our study.
To date, dosimetric studies considering anatomical variations have focused primarily on bladder volume. Siavashpour et al. [19,20] assessed the differences in doses received by other healthy organs by variations in bladder volume, and attempted to determine the optimal bladder volume. They categorized the bladder as empty (the bladder volume was unregulated after enema) or full (120 mL). CT images of empty and full bladders were obtained during each phase of bladder filling; dose contouring and planning were based on the GEC-ESTRO recommendations. The relative differences in rectum and sigmoid D2mL values by bladder status (empty vs. full) were not statistically significant (rectum D2mL: 0.7% ± 8.2%, p = 0.726; sigmoid D2mL: 0.3% ± 11.5%, p = 0.901). The cited authors then subdivided bladder volume into four categories, <70, 70–110, 110–170, and >170 mL, in reference to the absolute dose. The dose distributions to the bladder, rectum, and sigmoid colon were lowest when the bladder volume was <70 mL [20]. Sharma et al. [21] used the same volume categories to assess the effect of bladder volume on doses received by healthy organs; the bladder D2mL increased by approximately 7% when bladder volume exceeded 170 mL. The dose to the rectum was highest and the sigmoid D2mL greatest in the 70–110 mL subgroup [21].
Compared to previous studies (Table 5), the strength of our present study is that we considered how anatomical variations affected dose distributions. Previous studies have principally focused on bladder volume, with few studies on other organs. In addition, we identified certain parameters predicting doses of final 3D ICBT treatment plans by establishing criteria for various anatomical variations. In particular, bladder volume >250 mL indicated that 3D ICBT treatment planning would optimize dose distribution.
Our study had several limitations. The first is the small sample size, as we have only treated a small number of patients with 3D ICBT. Patients exhibiting cervical or parametrial invasion who were suitable for comparative dosing were selected; patients with advanced disease (such as disease featuring bladder invasion) were excluded. In addition, we divided all patients into subgroups consisting of even fewer patients, which compromised statistical power. The second limitation is that we did not study all known anatomical variations. Such variations are organ-specific; different criteria are used to evaluate each different organ. The overall anatomical variation of any patient is a combination of the variations in each organ. Despite these limitations, we suggest that that our study is meaningful, because we introduced a new concept.
In conclusion, previous studies on ICBT dose distributions by anatomical variations focused principally on the bladder, and information on other organs is lacking. We compared dose distribution of 2D ICBT and those of 3D ICBT by variations in the sigmoid colon, rectum, small bowel, and bladder. Of the pelvic anatomical variations studied, bladder volume over 250 mL was the most important in terms of reducing the dose to internal organs without affecting the target dose during 3D compared to 2D ICBT. Further prospective studies or larger size of studies would be required.
Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2018.00353
Table S1. Comparisons of dose parameters according to sigmoid circumference. Table S3. Comparisons of dose parameters according to bowel circumference. Fig. 1.Axial magnetic resonance imaging showing outlines of anatomical variations in the bowel and sigmoid. 
Fig. 2.Example dose-volume histogram (DVH) of the seven dose parameters measured. ROI, region of interest; GTV, gross tumor volume; HR-CTV, high-risk clinical target volume; IR-CTV, intermediate-risk clinical target volume. 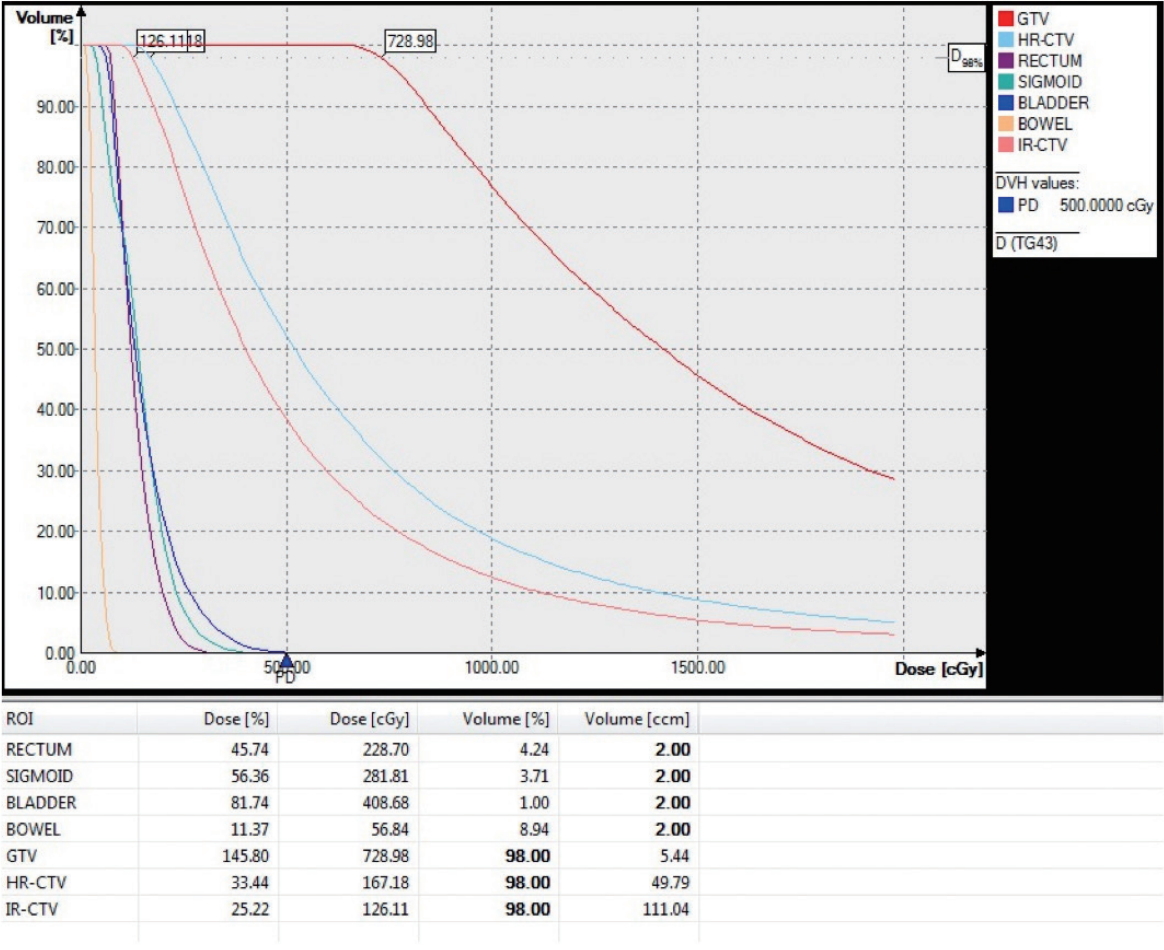
Table 1.Patients’ characteristics Table 2.Comparisons of dose parameters according to bladder volume Table 3.Comparisons of dose parameters according to sigmoid V3.5Gy
Table 4.Studies comparing 2D ICBT and 3D ICBT
Values are presented as mean ± standard deviation. Bold type was statistically significant factor. ICRU, International Commission on Radiation Units; N/A, not available. Table 5.Comparison of the effect of bladder volume on organ at risk with previous studies
References1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: cervical cancer [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; 2018 [cited 2018 Sep 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
2. International Commission on Radiological Units and Measurements. Dose and volume specification for reporting intracavitary therapy in gynecology (ICRU Report 38). Bethesda, MD: International Commission on Radiological Units and Measurements; 1985.
3. Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45.
4. Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77.
5. Hellebust TP, Kirisits C, Berger D, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol 2010;96:153–60.
6. Dimopoulos JC, Petrow P, Tanderup K, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol 2012;103:113–22.
7. Fellner C, Pötter R, Knocke TH, Wambersie A. Comparison of radiography- and computed tomography-based treatment planning in cervix cancer in brachytherapy with specific attention to some quality assurance aspects. Radiother Oncol 2001;58:53–62.
8. Montana GS, Fowler WC. Carcinoma of the cervix: analysis of bladder and rectal radiation dose and complications. Int J Radiat Oncol Biol Phys 1989;16:95–100.
9. Ogino I, Kitamura T, Okamoto N, et al. Late rectal complication following high dose rate intracavitary brachytherapy in cancer of the cervix. Int J Radiat Oncol Biol Phys 1995;31:725–34.
10. Kobayashi K, Murakami N, Wakita A, et al. Dosimetric variations due to interfraction organ deformation in cervical cancer brachytherapy. Radiother Oncol 2015;117:555–8.
11. Potter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 2018;9:48–60.
12. Derks K, Steenhuijsen JLG, van den Berg HA, et al. Impact of brachytherapy technique (2D versus 3D) on outcome following radiotherapy of cervical cancer. J Contemp Brachytherapy 2018;10:17–25.
13. Dimopoulos JC, Potter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol 2009;93:311–5.
14. Simha V, Rai B, Patel FD, et al. Clinical outcomes with MRI-guided image-based brachytherapy in cervical cancer: an institutional experience. Brachytherapy 2018;17:345–51.
15. Kang HC, Shin KH, Park SY, Kim JY. 3D CT-based high-dose-rate brachytherapy for cervical cancer: clinical impact on late rectal bleeding and local control. Radiother Oncol 2010;97:507–13.
16. Pelloski CE, Palmer M, Chronowski GM, Jhingran A, Horton J, Eifel PJ. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005;62:131–7.
17. Tan YI, Choo BA, Lee KM. 2D to 3D evaluation of organs at risk doses in intracavitary brachytherapy for cervical cancer. J Contemp Brachytherapy 2010;2:37–43.
18. Mazeron R, Champoudry J, Gilmore J, et al. Intrafractional organs movement in three-dimensional image-guided adaptive pulsed-dose-rate cervical cancer brachytherapy: assessment and dosimetric impact. Brachytherapy 2015;14:260–6.
19. Siavashpour Z, Aghamiri MR, Jaberi R, et al. A comparison of organs at risk doses in GYN intracavitary brachytherapy for different tandem lengths and bladder volumes. J Appl Clin Med Phys 2016;17:5–13.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |