Natural radioprotectors and their impact on cancer drug discovery
Article information
Abstract
Cancer is a complex multifaceted illness that affects different patients in discrete ways. For a number of cancers the use of chemotherapy has become standard practice. Chemotherapy is a use of cytostatic drugs to cure cancer. Cytostatic agents not only affect cancer cells but also affect the growth of normal cells; leading to side effects. Because of this, radiotherapy gained importance in treating cancer. Slaughtering of cancerous cells by radiotherapy depends on the radiosensitivity of the tumor cells. Efforts to improve the therapeutic ratio have resulted in the development of compounds that increase the radiosensitivity of tumor cells or protect the normal cells from the effects of radiation. Amifostine is the only chemical radioprotector approved by the US Food and Drug Administration (FDA), but due to its side effect and toxicity, use of this compound was also failed. Hence the use of herbal radioprotectors bearing pharmacological properties is concentrated due to their low toxicity and efficacy. Notably, in silico methods can expedite drug discovery process, to lessen the compounds with unfavorable pharmacological properties at an early stage of drug development. Hence a detailed perspective of these properties, in accordance with their prediction and measurement, are pivotal for a successful identification of radioprotectors by drug discovery process.
Introduction
The causes of serious ill-health in the world are changing. Infection as a major cause is giving way to non-communicable diseases such as cardiovascular disease and cancer [1]. Cancer it is one of the complex genetic diseases and remains leading cause of death globally. It involves multiple changes in gene expression leading to cell proliferation, deregulated balance, finally resulting abnormal cell growth producing malignant tumors with the potential to invade or spread to other parts of the body [2]. Faster the tumor’s growth rate the more rapidly its cells are multiplying. The International Agency for Research on Cancer (IARC) by survey approximately reported nearly 12.7 million new cancer cases per year and 7.6 million deaths reported worldwide [3] and by 2020 it is predicted to be 12 million deaths and 20 million new cases of cancer. The past decade has witnessed a considerable progress in understanding the hallmarks of cancer causes, along with advances in early detection and various treatment modalities.
Radiotherapy is used as a primary treatment modality in the cure of cancer [4,5]. The use of radiotherapy began early in the 20th century, prior to chemotherapy, and preceding the wide-scale use of randomized clinical trials, to determine the effectiveness of medical treatments [6].
Eighty percent of cancer patients need radiotherapy during the course of treatment, either for palliative or curative purpose. Of the approximately 1.4 million cancer patients; one million people will be undergoing radiation. Each year around 10.9 million people pronounced with cancer worldwide, amongst them around 60% people are instructed for radiotherapy treatment and 40% of them go for treatment with curative intent [7]. This is because of its cost, accounting for only 5% of the total cost of cancer cure. In the course of treatment, radiation produces various biological perturbations in cells; as normal cell toxicity limits the doses used in effective treatment; methods are designed to strike a balance between eliminating cancer cells and protecting normal tissues. Hence the goal of radiation therapy is to achieve maximum tumor cell killing while minimizing injury to normal tissues (therapeutic ratio). Local failure of tumor suppression is the cause of 40%–60% of cancer deaths and may occur in 60%–80% of cancer patients. Therefore, modulation of therapeutic index has been a central issue in radiotherapy for decades [8].
Unfortunately as long as acute toxicity occurring in the interim of clinical radiotherapy, higher radiation doses cannot be used which would be more effective. Hence, improving the therapeutic ratio to reduce these toxicities (that is ratio of normal tissue toxicity to cancer cell killing), there is a need for tremendous research interest in search of radioprotective drugs. It is ultimately the use of radioprotectors to protect normal tissue and radiosensitization of cancerous tissue that limits the ability to maximize patient’s toxicity free treatment for survival.
Radiosensitizers are mainly used for radiosensitizaton of tumour cell. Radiosensitizers are compounds that when combined with radiation, sensitizes the tumor cells achieving greater tumor inactivation by apparently promoting the fixation of the free radicals produced by radiation damage at the molecular level [9]. On the other hand, radioprotectors are used to protect normal cells; these are the compounds that are designed to reduce the damage in normal tissues caused by radiation. These compounds are often antioxidants and must be present before or at the time of radiation for effectiveness. Other forms of agents are also present termed mitigators, which are mainly used to minimize toxicity even after radiation has been delivered [10]. In spite of more than six decades of research on the development of radioprotectors, there is no safe and effective nontoxic radioprotector available for human use [11]. This has enthused extensive search to find effective and nontoxic radioprotectors. Hence the interest has shifted towards the use of natural products in radioprotection.
Radioprotectors
On the other hand, radioprotective agent or radioprotector could be a chemical or a drug that reduces the radiation elicited damages, once administered to living organisms. Identification of an efficient and nontoxic radioprotector is a vital goal for radiation oncologists and basic radiation biologists. The most protective radioprotectors developed so far are aminothiols and their derivatives viz. cysteamine, aminoethanisothiuronium bromide hydrobromide (AET) and amifostine (WR-2721). Some of these compounds have been used to prevent complications of radiation therapy successfully in cancer patients and have conjointly been thought to protect against radiation hazards in clinical use and in accidental radiation exposure scenarios [12]. Bourhis and Rosine [13] found that amifostine in specific is found to be effective in patients with head and neck squamous cell carcinoma, but use of amifostin in radiotherapy is limited because of its toxicity like nausea, sneezing, diarrhea, sleeplessness, hypotension, dizziness, hypocalcemia, and hiccoughs [14]. Regardless of its current clinical applications, amifostin has not been approved for use in any clinical nuclear/radiological exposure setting. The negative effects of amifostine encompasses; cost, toxicity, limited protection of the central nervous system, limited routes of administration and narrow time windows [15]. Another synthetic compound is 5-aminosalicylic acid (5-ASA) was studied by Lans [16] who observed that on pretreatment of 5-ASA, significant reduction in the micronuclei formation up to 40%–50% when compared to radiation control, with a dose modification factor (DMF) 2.02–2.53. Sulfasalazine (SAZ) yet another compound optimally protected mice on treatment at 120 mg/kg, without any toxicity. At this dose, SAZ protected plasmid DNA (pGEM-7Zf) against Fenton reaction-induced breaks, suggesting free radical scavenging as one of the possible mechanism for radioprotection but this too was found to have some level of toxicity [17]. Practical applicability of majority of these synthetic compounds remained limited owing to their high toxicity [18]. An ideal protector is one which gives a high degree of protection to normal tissues, with little or no protection to tumor cells and most importantly, should be nontoxic.
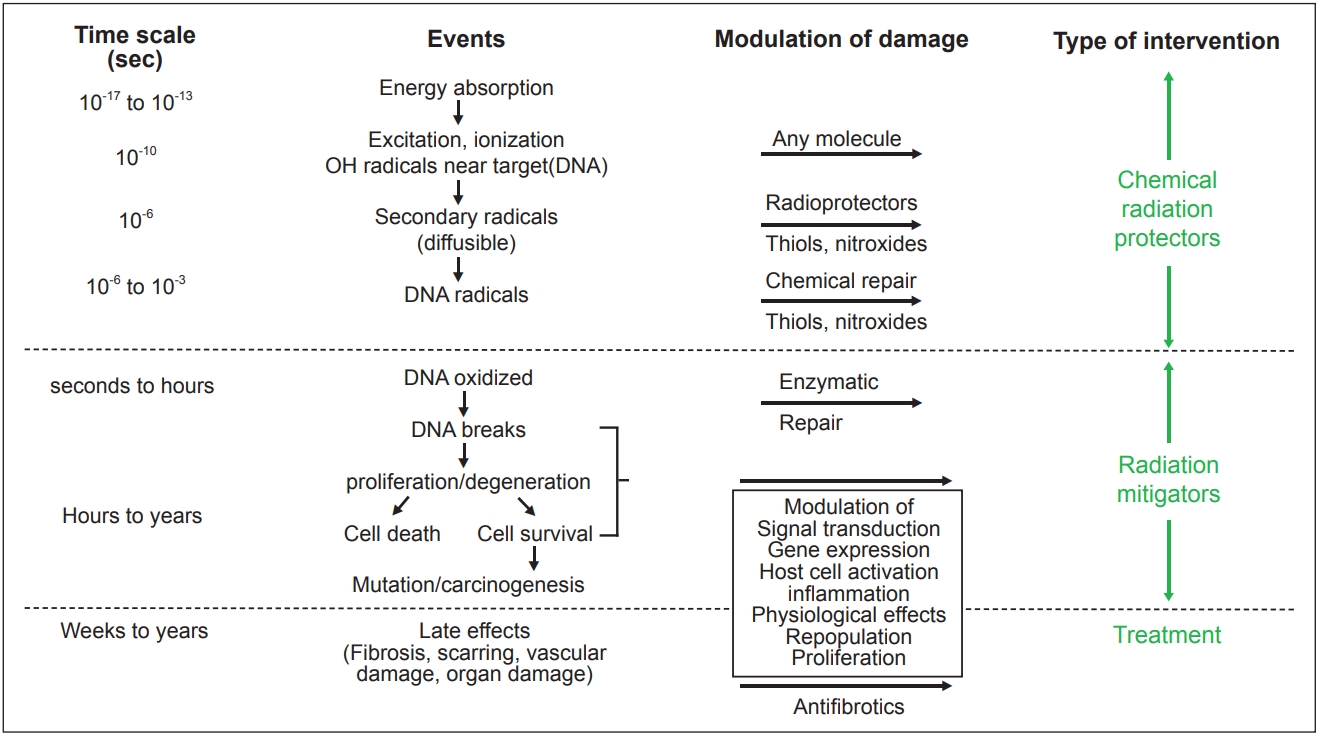
An understanding of the events occurring during and shortly after irradiation of tissues and cells is important to understanding the mechanism of action of radioprotectors and mitigators. Fig. 1 shows the sequence of events in cells and tissues following radiation exposure.
The failure to obtain more effective and less toxic radioprotectors from synthetic compounds prompted researchers to focus towards evaluating the radioprotective potential of natural products [29,30].
Natural Radioprotectors
Natural radioprotectors are plant compounds that protect normal (noncancerous) cells from the damage caused by radiation therapy. Natural plant products are nontoxic with proven therapeutic benefits and have been utilized since ancient times for curing various ailments. About 60% of the 1,184 new drugs developed over the past 25 years owe their origin to natural sources. Till today, nearly 74 plant products have been screened for their radioprotective potential in various in vitro and in vivo studies. The use of herbals and dietary modulators in combination with radiation have enhanced tumor killing by radiosensitizing tumor cells in turn protection normal cells against radiation [31].
Firstly discussing about radiosensitizers, the use of certain plants as radiosensitizers has been reported in literature. Reports have shown that Ayurvedic formulation, triphala obtained from combining three plants, i.e., E. officinalis, T. bellirica, and T. chebula, in combination with gamma radiation lead to radiosensitization of tumor cells, MCF-7 breast cancer cell line. It was further found that triphala spared normal cells, such as mouse hepatocytes and spleen cells at concentrations that were toxic to MCF-7 [32], by activating pro-apoptotic signals in neuroblastoma xenografts. Similarly Azadirachta indica leaf extracts exhibited radiosensitizing effect when exposed to single (10 Gy) or fractionated (2 Gy/day for 5 day) doses of radiation [33]. Ziziphus mauritiana anther plant extracts studied by Bache et al. [34] enhanced cellular toxicity with decreased clonogenic survival in combination with radiation (4 Gy) in head and neck squamous carcinoma cell line and under hypoxic condition it induced cytotoxicity and radiosensitivity in glioma cells. An ideal radiosensitizer for use as an adjunct in radiotherapy should have such characteristics as low toxicity, high radiosensitizing efficiency for hypoxic cells, least effect on normal cells and minimum interference with other therapies. It should also be economically affordable.
On the other hand, radioprotectors from plant source have been studied to protect normal cells. For example the plant Pilea microphylla, protected livers of irradiated mice from depleting endogenous antioxidants like superoxide dismutase (SOD), glutathione, thiols and catalase. It conferred overall radioprotection by protecting the gastrointestinal and hematopoietic system as studied by Bansal et al. [35]. Some of the herbal plants used as radioprotectors are listed in Table 1.

List of herbal plants used as radioprotectors and their uses in radioprotection during cancer radiotherapy
At the same time large number of phytochemicals obtained from plant sources has been reported to be radioprotective activity in various animal models. All these reports support the argument that plant products and their isolates have great potential to be developed as radioprotectors. Speaking about constituents of plant products, plants are rich sources of polyphenols which include anthocyanins, flavonoids, stilbenes, tannins, lignins, etc. [36]. Among these, plant phenolic compounds such as lignin precursors and flavonoids are the important constituents of the human diet [37]. These compounds have been recognized as beneficial antioxidants that can scavenge harmful active oxygen species.
Antioxidant and anti-inflammatory properties of curcumin in mouse models are well documented by Conney et al. [38]. Lupeol has been examined for its cardioprotective effects and was determined to provide 34.4% protection against in vitro LDL oxidation [39]. Lycopene another compound is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits and vegetables, in line with one preliminary study, consumption tomato paste for 3 months decreases sun injury by UV radiation by 30 minutes through the action of lycopene. Resveratrol is a stilbenoid, a polyphenolic compound found in grapes, red wine, purple grape juice, peanuts, and some berries. Xanthorrhizol may be a sesquiterpenoid compound extracted from Curcuma xanthorrhiza was found to possess antibacterial, anticancer and anti-inflammatory activity. The stem has additionally been used to treat inflammation in postpartum uterine bleeding. Recent studies also proved that it possess nephroprotective activity. It is also worth mentioning that phytochemicals have the advantage of low toxicity, therefore, they might be more easily and safely used in patients undergoing radiotherapy than other radioprotective chemicals. Based on the present status of herbal radioprotectors (Table 2), the future holds promise for revealing the potential natural products in radioprotective drug discovery.
Drug Discovery Process
The search for potent radioprotectors from plant source can be overwhelmed by use of drug discovery process. It is a process of generating new compounds that is targeted towards disease. The drug can be identified by comprehensively understanding disease process. It is a challenging process with significant trial and error element. It is indeed an expensive process, requiring 12–15 years and millions to dollars to designing a drug and to reach the market from its initial discovery stage [40]. Until the recent few decades, the science of drug discovery has relied heavily upon systematic screening of the drug related to disease process. This eventually paved way to increased usage of medicinal chemistry and allied disciplines wherein the chemistry of the potential drugs were understood and exploited in great detail to design more effective molecule. In fact, there are a number of drugs in current clinical use, which have emerged out of traditional medicinal chemistry approaches involving organic synthesis of potential drug candidates and pharmacological testing. For example, the taxanes [41], paclitaxel, and docetaxel [42] has been show antitumor activity against breast, ovarian and other tumor types in the clinic trial. Paclitaxel stabilizes microtubules and leading to mitotic arrest [43]. In addition, the camptothecin derivatives irinotecan and topotecan, have shown significant antitumor activity against colorectal and ovarian cancer, respectively [44]. The scenario is, however, changing rapidly and the science of drug discovery has witnessed several paradigm shifts [45]. Many of these can be attributed to advances in molecular biology, delineation of the molecular bases of pathological processes, as well as those of drug actions in many cases, leading to shifts in the discovery focus from a ligand lead to the target molecule. With the advancement in the high throughput screening techniques [46] and the genomics and post-genomics eras to analyze whole genomes and proteomes, are helpful in providing huge amount of information, not only with respect to the genome sequences and protein structures but also with respect to regulation, gene-expression, and protein–protein interactions (PPIs). The availability of such information in publicly accessible databases and the advances in both computing power as well as in computational methods for data mining and modeling, have led to the emergence of several in silico approaches to systematically address several questions in biology, with an obvious impact on drug discovery. Systems level approaches to discover drug aid at multiple stages in the drug discovery pipeline, particularly in target identification and in identifying the molecular basis of disease for rational drug discovery [47]. Target selection or identification involves choosing a disease to treat. This means selection or discovery of biological targets such as receptors or particularly enzymes or ion channels linked to a pathological process. Identification and validation of drug able targets from thousands of candidate macromolecules are still a challenging task [48]. Numerous technologies for addressing the targets have been developed recently. Genomic and proteomic approaches are the major tools for target identification. For example, a proteomic approach for identification of binding proteins for a given small molecule involves comparison of the protein expression profiles for a given cell or tissue in the presence or absence of the given molecule. This method has not been proved very successful in target discovery because it is laborious and time consuming [49]. Therefore complementary to the experimental methods a series of computational approach have also been developed for drug target identification, which may speed up the whole biological/chemical/medical community, and lead to the high-throughput, low cost, and the means to save time and energy [50]. Currently, microarray technology is used to identify disease markers, biological processes and novel transcriptional cascades. This technology represented as one of the first functional genomics platforms that exploit genome sequence data to analyze a biological process (gene transcription) on a gene-by-gene basis. However, this tool is also facing some problems such as consistency of experimental results [51] and also data comparison obtained from different platforms.
To overcome such kind of flaws, in silico approaches for studying PPI are gaining importance in identifying drug targets. In view of these Hormozdiari et al. [52] developed a strategy for identifying potential multiple-drug targets in pathogenic PPI networks with the goal of disrupting known pathways/complexes and showed how protein interaction networks are disrupted on altering the hub protein so as to maximize the number of potential pathways/complexes. Finally 28 potential targets were identified (four of them known drug targets) on the E. coli PPI network whose removal led to partitions of network in to two sub-networks with relative sizes of 1 to 5.
After target identification, its validation becomes important criteria; the next step is to develop a therapy that affects the target in a way that interferes with its ability to promote cancer cell growth or survival. It involves demonstration of relevance of the target protein in a disease process. For example, a targeted therapy could reduce the activity of the target or prevent it from binding to a receptor that it normally activates, among other possible mechanisms. Validation of targets becomes important because, during clinical trials, the target validation fails for about 50% of therapeutic approaches. A retrospective analysis of drug development programs at Pfizer revealed some opportunities for optimization of the drug development process. To overcome such a failure three knowledge pillars have been identified, which increase the likelihood of candidate survival in phase II trials such as: deep understanding of the drug exposure at the site of action, target binding of the drug, and clear expression of functional pharmacological activity. The latest reached highest significance for prediction of success in clinical trials. Hence, an in-depth biological understanding of a molecular target as one of the very early steps in the entire drug discovery and development process which can determine later success or failure of the emerging drug candidate is required.
Tens of thousands of potential drug substances (obtained from massive compound libraries) are tested against the target proteins in a robotic process called high-throughput screening (HTS). The identification of radiation-protecting agents is an important goal for radiation oncologists and basic radiation biologists. Herbal plants are gaining prime importance in search of radioprotecting agents.
Virtual screening has reached a status of a dynamic and lucrative technology in probing for novel drug-like compounds (radioprotectors) or so-called hits in the pharmaceutical industry [53]. Various physiochemical descriptors, e.g., polar surface area, predicted pharmacokinetic properties (ADME), passive transcellular permeability in the intestine or in the brain, are used to filter out these compounds. ADME processes play a critical role in determining the inclination of a drug candidate, and thus its therapeutic efficacy. The initial analysis of ADME properties, e.g., anesthetic agents in the late nineteenth century, focused on the partition coefficient (logP) between water and oil, basically the lipophilicity of the compound. This has served as one of the fundamental principles for drug discovery and design [54]. Many drugs are affected by lipophilicity, highly lipophilic compounds have low solubility and poor absorption. Increase in the lipophilicity increases the probability of binding to hydrophobic protein targets rather than desired one leading to toxicity [55]. First-phase metabolism influences oral bioavailability and toxicology of drugs Once compounds enter the bloodstream, they need to get to the target site, that is, the site(s) of malignancy. The initial interaction of the compounds will be with plasma protein, which exerts a large influence on the distribution process. The rapid elimination of active compound from the site of action (or) from the body itself can severely impact the efficacy of any therapeutic agent. The most common routes of elimination are via renal and/or biliary excretion, the kidney being the most important organ. Once the knowledge of the target is available, rapid docking algorithms are used to place the available candidate compounds within the active site of the biochemical target of interest and then the activity of compounds is rank ordered by analyzing the steric and electrostatic components [56].
Docking and scoring would then be applied only on these compounds that meet these filtering criteria [57].
The control of cell growth and its behavior has long been recognized to be complex. However, we are increasingly daunted by exactly, how great a challenge it will be to understand or predict normal cell behavior and the rewiring that goes on in cancer cells. There are some recent examples in which the discovery of entire levels of cellular regulation, such as microRNAs, adds yet another layer of complexity. Radiotherapy one of the treatments for cancer, faces a major drawback as it inevitably involves exposure of normal tissues to the deleterious effects of ionizing radiation. Damage to DNA and membrane lipids, is the critical factors in radiation-induced cellular damage and reproductive cell death [58]. The severe side-effects of radiotherapy and chemotherapy prohibit the application of doses which are high enough to kill all cancer cells, which in turn leads to the development of radio- and chemoresistance cancerous tissue. Many strategies have been proposed to improve radiotherapy, most of which are based on two main concepts: firstly, the sensitization of tumour tissues for radiation, which allows radio-resistant tumor populations to be killed and/or radiation doses to be reduced [59]. Secondly, the protection of normal tissues from radiation damage by radioprotectors.
Radioprotector is the prime antidote to radiation injury and until now none radioprotector has undeniably reached the stage of drug development. Hence to find new radioprotective agents, an attractive approach is to repurpose the protracted list of accepted non radioprotective drugs, i.e., by computational approach one example is by comparing publicly available gene expression data of ionizing radiation-treated samples from the Gene Expression Omnibus (GEO) database with gene expression signatures of more than 1,309 small-molecule compounds from the Connectivity Map (cmap) dataset one can discover new radioprotectors in silico [60].
Conclusion
Drugs are designed for a particular purpose or against a specific protein target; still they interact with different proteins showing their off-targets. This sometimes results in side effects for a particular drug. This kind of interaction of a drug with different proteins is termed as ‘drug polypharmacology’ [61]. Drugs used for some other purpose, if show association of some new activity, can therefore be repurposed for this new activity. In particular, if a drug in the market shows new target association it can be used for that new activity as well. This phenomenon is termed as ‘drug repositioning or repurposing’. In silico approaches could be adopted as the primary step for attaining such processes, which fasten sorting out potentially positive molecules for a given end point. Such approach may involve structural and/or functional studies at virtual level [62]. The drug repurposing strategy by connecting the GEO data and cmap can be used to identify known drugs as potential radioprotective agents.
Thus, the protection of humans and animals from the influence of infrared radiation is a major challenge in radiation biology and medicine. It has been suggested that various chemical structures may protect against the cell and tissue toxicities and delayed carcinogenesis that are induced by radiation.
Hence there is a need for understanding the mechanism of radiation damage and its possible prevention by drugs such as radioprotectors. Herbal medicines have been gaining importance in radioprotective drug discovery owing to lesser side effects as reviewed extensively by many authors. In silico approach adds up in screening and identification of potent radioprotectors.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors thank the Registrar, Vignan’s Foundation for Science, Technology, and Research (Deemed to be University) for support in developing this review article.