Introduction
In South Korea, the incidence of patients with brain metastasis from all malignancies is 5.0 per 1,000 person-years, and the order of major primary sites based on decreasing frequency is lung, liver, breast, and colon [1]. Whole brain radiotherapy (WBRT) is widely used for palliative aim in patients with multiple brain metastases and for prophylactic aim in patients with small cell lung cancer in complete remission after the primary treatment [2-7]. Because the outcomes of patients treated with WBRT is generally poor, the toxicity has been neglected. However, survival is longer owing to the development of cancer treatment and the quality of life is become important [8].
The use of parallel opposed fields for WBRT is a simple and effective method to cover the entire brain. Computed tomography (CT) simulation enables the dose distribution of normal organs to be evaluated, although it did not change significantly from two-dimensional radiotherapy (2D-RT) to three-dimensional conformal radiotherapy (3D-CRT). It also made for us to find the large amount volume of parotid glands irradiated during WBRT. Noh et al. [9] recently demonstrated through a normal tissue complication probability model that the parotid gland should be considered as an organ at risk (OAR) during WBRT.
Several researchers have attempted to reduce the dose of the parotid gland, primarily by modifying the lower margin of the treatment field [10,11]. Modifying the field has shown good results in protecting the parotid glands, but the target coverage is unclear. We have attempted to reduce the irradiated dose of the parotid gland by adding a superior anterior beam and demonstrated that it could block the parotid gland effectively without jeopardizing the target coverage [12]. However, using a non-coplanar beam could render the treatment process cumbersome. Inspired by this technique, we applied a four-field box technique for WBRT after a patient generated a posture with his head forward. The aim of this study is to evaluate the efficacy and feasibility of four-field box WBRT (FB-WBRT) by comparing it with bilateral WBRT (B-WBRT).
Materials and Methods
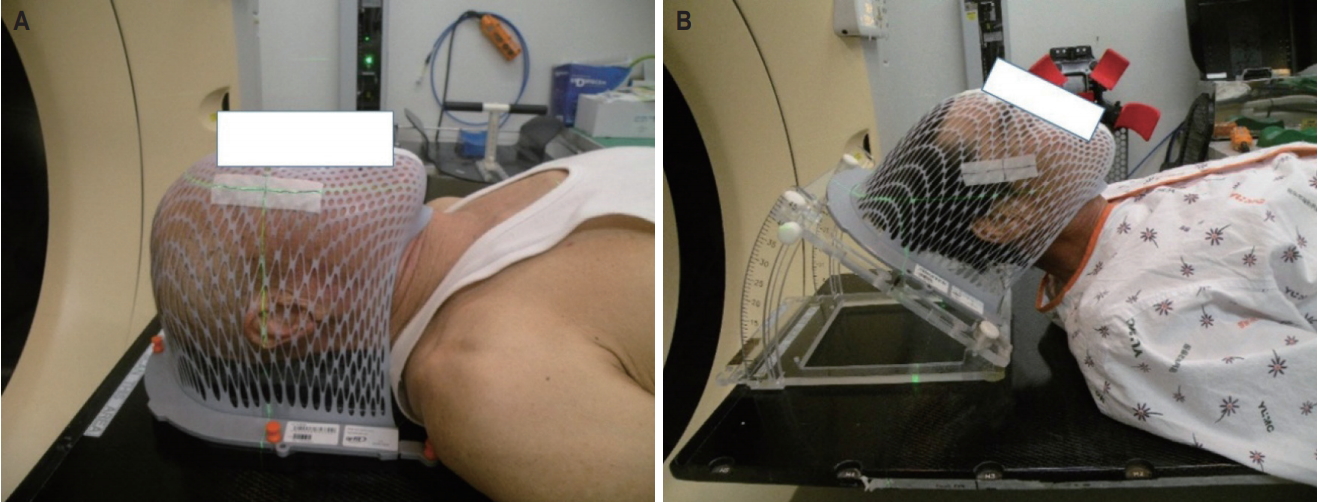
Between March 2016 and September 2018, 20 patients with brain metastases underwent WBRT using the four-field box technique. The informed consent was waived. The median age of the patients was 61 years (range, 47 to 87 years). The primary tumor was from lung cancer (non-small cell lung cancer in 12 patients and small cell lung cancer in 7) and one patient had breast cancer. Two CT simulations per person were performed. One was in the traditional supine position for B-WBRT and the other was applied with a tilting acrylic supine baseplate (MedTec-Civco, Orange City, IW, USA) to elevate his head by 40° for FB-WBRT, using a thermoplastic mask for immobilization (Fig. 1). We obtained the CT scan images of 5-mm slice thickness, and contoured the OARs, including both the parotid glands and lenses. The brain contours were identified by auto-segmentation of the Eclipse Treatment Planning System 8.6 (Varian Medical System, Palo Alto, CA, USA). The clinical target volume (CTV) included the brain parenchyma and the spinal cord up to the lower level of the atlas, as has expanded by 5 mm in all directions to create the planning target volume (PTV).
B-WBRT plan was subsequently generated using the field-in-field technique for reducing the maximum dose, and to increase dose homogeneity [12]. The beam direction for FBWBRT consisted of anterior, posterior, and bilateral beams (Fig. 2). Enhanced dynamic wedge was applied in the anterior and posterior fields to compensate for skull convexity. A suitable wedge angle of 15º to 30º was selected for each patient. According to our institutional practice, WBRT was administered using the schedule of 30 Gy in 10 fractions, for 5 days a week. Electronic portal imaging was performed to obtain images on the anterior and right at the first and 6th days of treatment.
The conformity index (CI) and homogeneity index (HI) were utilized in the treatment plan analysis. The CI is defined as the ratio of the PTV that receives 95% of the prescribed dose to the entire PTV, while the HI is the ratio of the maximum target dose to the prescribed dose. Wilcoxon-signed rank tests were used to compare the dosimetric outcomes, including the dose coverage and OAR doses between the B- and FB-WBRT plans. All the statistical analyses were performed using the SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) software package.
Results
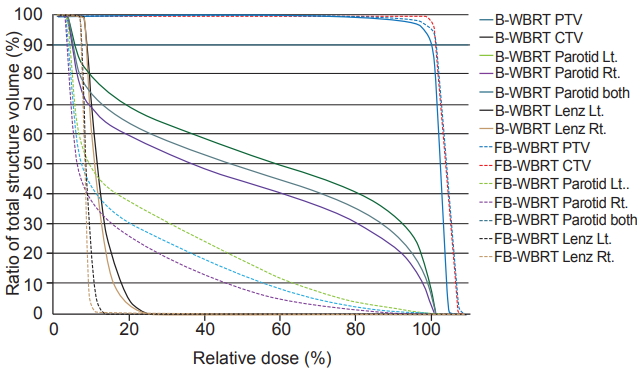
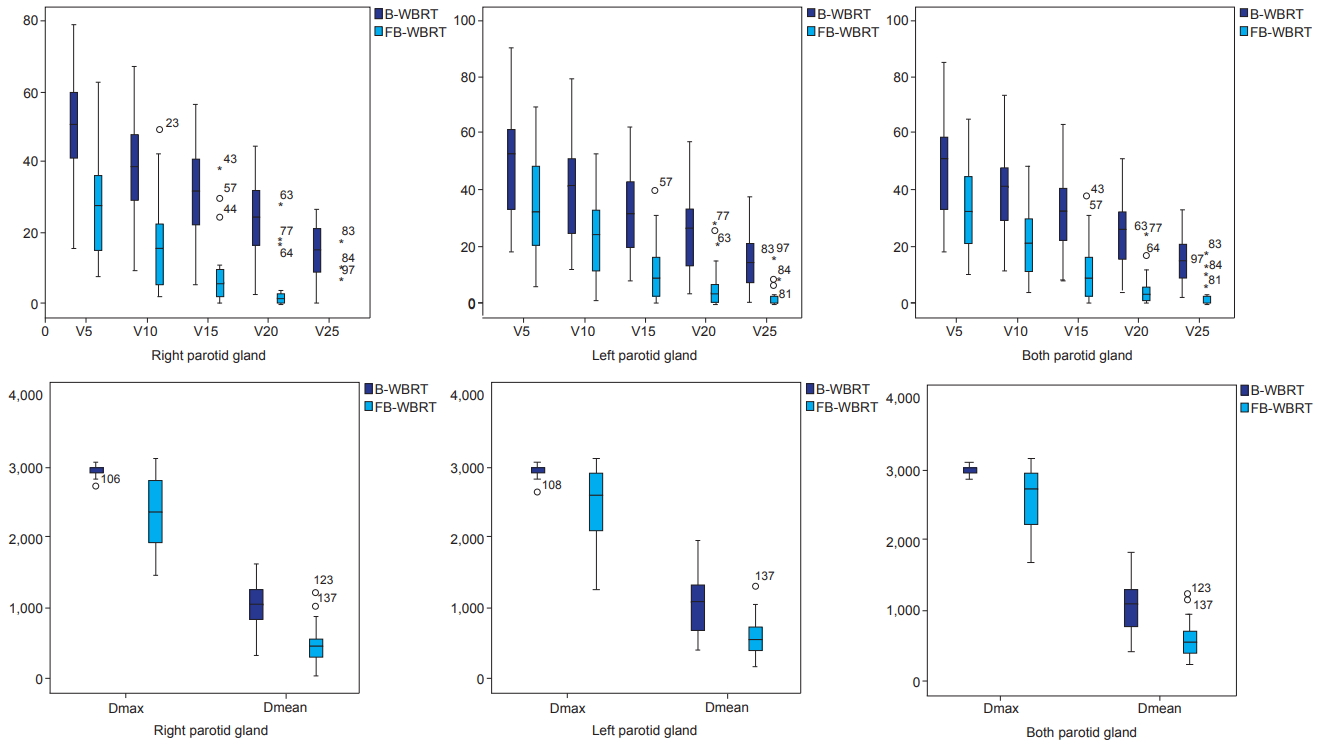
The mean volume of the right parotid, left parotid, and both parotids were 29.7 ± 13.7 cm3, 28.1 ± 11.2 cm3, and 57.8 ± 25.1 cm3 in B-WBRT, respectively; 29.5 ± 14.6 cm3, 28.4 ± 12.5 cm3, and 58.0 ± 26.6 cm3 in FB-WBRT, respectively. The dose-volume statistics of the parotid glands are summarized in Table 1, and Fig. 3 demonstrates the dose-volume histogram (DVH). The Dmean of both parotid glands is 10.2 Gy (range, 3.8 to 17.8 Gy) in B-WBRT and 5.4 Gy (range, 2.0 to 11.7 Gy) in FBWBRT (p < 0.05). Compared to B-WBRT, FB-WBRT reduced the mean dose of the right and left parotid glands from 10.1 Gy to 4.9 Gy and from 10.4 Gy to 5.8 Gy, respectively (p < 0.05). Further, V5, V10, V15, V20, and V25 for the parotid gland decreased significantly in FB-WBRT (p < 0.05) (Fig. 4).
The Dmax of the lens was 7.3 ± 3.4 Gy on the right and 7.6 ± 4.6 Gy on the left in B-WBRT, and 5.0 ± 2.3 Gy on the right and 5.0 ± 2.3 Gy on the left in FB-WBRT (p < 0.05). The Dmean of the lens was 4.2 ± 2.1 Gy vs. 3.0 ± 1.1 Gy on the right, and 4.2 ± 2.1 Gy vs. 3.0 ± 1.1 Gy on the left in the B-WBRT and NCWBRT plans, respectively (p < 0.05).
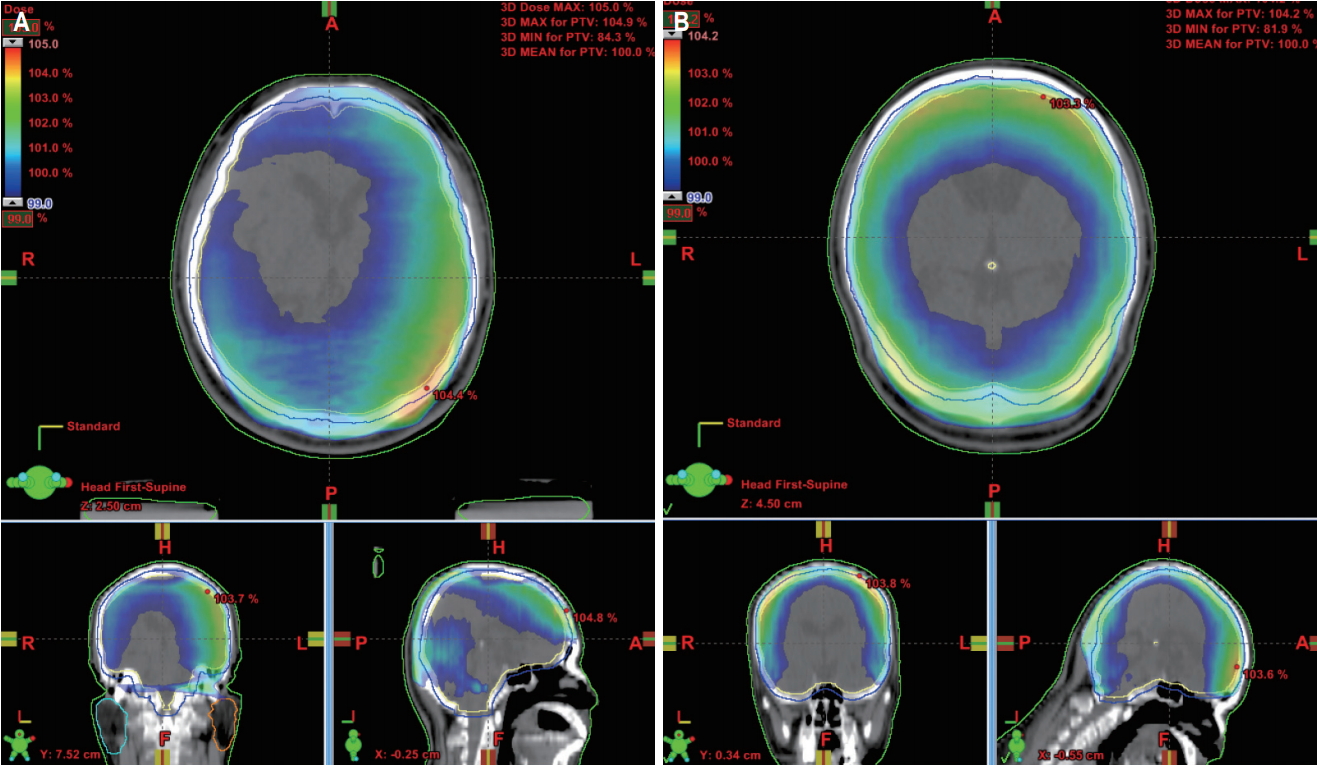
The dose distribution of both plans is shown in Fig. 5. The CI in B-WBRT and FB-WBRT were 0.86 ± 0.06 and 0.85 ± 0.05, respectively. The HI was 1.05 ± 0.01 in both plans. There was no statistical significance between both plans.
Discussion and Conclusion
Parotid sparing has been a major concern in radiation therapy for head and neck cancer because xerostomia is the most typical and uncomfortable side effect. The impairment of parotid gland function could cause problems such as poor dental hygiene, oral infections, and difficulties in chewing and swallowing [13,14]. To avoid severe xerostomia, the Quantitative Analyses of Normal Tissue Effects in the Clinic guidelines recommend that the mean dose to at least one parotid gland should be less than 20 Gy, or that the mean dose to the combined volume of both glands should be less than 25 Gy [15].
The use of parallel opposed fields is the most used method because it provides good target coverage. It includes the brain tissue, skull, and spinal cord to the lower level of the atlas. However, Trignani et al. [16] compared 2D-RT with 3D-CRT for WBRT and discovered that 28% of patients received a mean dose of >20 Gy in the parotid gland. Meanwhile, Noh et al. [9] reported that mean parotid doses of ≥20 and ≥25 Gy were observed in 34.4% and 6.3% of 64 individual glands, respectively. Burlage et al. [17] reported significantly reduced salivary flow rates during the first 2 weeks of RT. The flow rate has decreased by approximately 80% of the initial flow; subsequently, the total cumulative dose was 20 Gy. Given the typical treatment schemes of WBRT that is 30 Gy in 10 fractions, it appears necessary to make attempt to reduce the parotid dose for WBRT.
Efforts have been made to reduce the irradiation dose of the parotid gland. Fiorentino et al. [11] reported excellent outcomes in which the medians V20 and V25 of the right parotid gland were 3.5% (range, 0% to 44.5%) and 1.85% (range, 0.32% to 18%), respectively; further, for the left parotid gland, the medians V20 and V25 were 3.1% (range, 0% to 44.5%) and 1.8% (range, 0% to 32.2%), respectively. They have reduced the treatment field to encompass only the brain tissue and skull. Cho et al. [10] demonstrated the effectiveness in protecting the parotid gland using a modified field technique that is customized to a lower margin around the parotid gland region. The mean dose of the parotid gland was 17.4 Gy vs. 8.7 Gy, and the V20 was 48.4% vs. 18.2% in the conventional and modified fields, respectively.
Our previous study using non-coplanar beams demonstrated satisfactory results in which the Dmean of the parotid gland was 13.7 Gy and the V20 was 36.4% in non-coplanar WBRT without compromising the treatment field [12]. Although WBRT with non-coplanar is effective in sparing the parotid gland, we have devised a simpler method that yields the characteristics of the non-coplanar beam. By elevating the patient’s head, the anterior beam exhibits a non-coplanar-like effect and the posterior beam is available for use. We anticipated that the dosimetric outcomes in FB-WBRT to be similar to that of WBRT using non-coplanar beams. However, the average of Dmean and the V20 of the parotid gland were 5.4 Gy and 4.9% in FB-WBRT, respectively. These results are better than our expectations. These differences can be attributed to the difference in the PTV margin. This study used a 5-mm margin to create the PTV while our previous study used a 7-mm margin to create the PTV. These changes appear to exhibit a similar effect to the modified field of Cho’s study. Through the dosimetric outcomes of B-WBRT, this assumption is reasonable.
As shown in Fig. 5, FB-WBRT demonstrated a relatively uniform dose distribution in the brain region. However, there was no significant difference in the CI and HI. These results were caused by the out-region of the PTV in the posterior neck region being irradiated in FB-WBRT and the field-in-field technique that reduced the hot region in B-WBRT. The Dmax at point of B-WBRT was showed comparable with that of FBWBRT in our study (104.3% ± 0.95% vs. 104.9% ± 0.70%; p = 0.06).
Intensity-modulated radiotherapy (IMRT) has been widely used in head and neck cancer as it has been proven effective by several prospective trials and systemic reviews for parotid preservation [18-22]. The use of IMRT in WBRT is primarily focused on hippocampal sparing for preserving a patient’s neurocognitive function [23-25]. To the best of our knowledge, studies on parotid gland sparing in WBRT using IMRT are scarce. Considering the characteristics of IMRT, the use of IMRT for WBRT could decrease the irradiated dose to the parotid gland. IMRT is generally required a higher workload than 3D-CRT. Recently, the development of RT technique has shortened the time required for treatment planning, quality-assurance and beam delivery and could be overcome the drawback of IMRT [26-28]. However, the advanced RT technique has increased cost [29,30]. Therefore, 3D-CRT is advantageous in terms of cost-effectiveness and it is meaningful attempt to reduce the dose for the parotid gland in 3D-CRT for improving the patient’s quality of life.
In conclusion, a recent study has shown that xerostomia occurred significantly at the end of WBRT and appeared to be persistent [31]. Therefore, an effort to minimize parotid gland damage in WBRT is necessary. Compared to B-WBRT, FB-WBRT with a tilting acrylic supine baseplate was a simpler and more effective method to take feature of non-coplanar beam for protecting the parotid gland.