Maximum standardized uptake value at pre-treatment PET in estimating lung cancer progression after stereotactic body radiotherapy
Article information
Abstract
Purpose
This study aimed to identify the feasibility of the maximum standardized uptake value (SUVmax) on baseline 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET/CT) as a predictive factor for prognosis in early stage primary lung cancer treated with stereotactic body radiotherapy (SBRT).
Materials and Methods
Twenty-seven T1-3N0M0 primary lung cancer patients treated with curative SBRT between 2010 and 2018 were retrospectively evaluated. Four patients (14.8%) treated with SBRT to address residual tumor after wedge resection and one patient (3.7%) with local recurrence after resection were included. The SUVmax at baseline PET/CT was assessed to determine its relationship with prognosis after SBRT. Patients were divided into two groups based on maximum SUVmax on pre-treatment FDG PET/CT, estimated by receiver operating characteristic curve.
Results
The median follow-up period was 17.7 months (range, 2.3 to 60.0 months). The actuarial 2-year local control, progressionfree survival (PFS), and overall survival were 80.4%, 66.0%, and 78.2%, respectively. With regard to failure patterns, 5 patients exhibited local failure (in-field failure, 18.5%), 1 (3.7%) experienced regional nodal relapse, and other 2 (7.4%) developed distant failure. SUVmax was significantly correlated with progression (p = 0.08, optimal cut-off point SUVmax > 5.1). PFS was significantly influenced by pretreatment SUVmax (SUVmax > 5.1 vs. SUVmax ≤ 5.1; p = 0.012) and T stage (T1 vs. T2-3; p = 0.012).
Conclusion
SUVmax at pre-treatment FDG PET/CT demonstrated a predictive value for PFS after SBRT for lung cancer.
Introduction
Stereotactic body radiotherapy (SBRT) is used for curative treatment when surgery is not possible in patients with early stage lung cancer [1]. SBRT may be considered a first-line treatment modality for elderly patients with other serious diseases, such as restrictive/obstructive lung disease or double primary cancer, or for patients who are postoperative resection margin-positive or exhibit suspicious local recurrence [2]. SBRT, a hypo-fractionated high-dose radiotherapy (RT) introduced by Timmerman et al. [1] in the late 2000s, has dramatically altered contemporary methods of RT.
SBRT has demonstrated its ability to control tumors more effectively than conventional fractionated RT in cases with lower tumor burden. In addition to direct cell killing by radiation, SBRT also kills tumor cells in indirect ways, such as collapse of the tumor vascular structure and altering the tumor microenvironment [3]. In primary lung cancer, the outcome of SBRT is fairly good, affording the same level of local control as surgery. However, the proportion of distant metastasis is higher than that of surgery [4]. Thus, SBRT has not been able to completely replace lobectomy, despite the advantages in quality of life in patients with early stage non-small cell lung cancer.
In the era of SBRT, the role of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET/CT) has emerged. In terms of detecting cancer, FDG PET/CT can be used to confirm the therapeutic response of SBRT or to confirm relapse [5,6]. Additionally, Clarke et al. [7] reported that the maximum standardized uptake value (SUVmax) in primary FDG PET/CT can be used to assess prognosis. These results demonstrated that progression-free survival (PFS) and distant metastasis-free survival could be affected by the metabolic activity of the tumor before SBRT [7].
In this study, we aimed to analyze the relationship between the SUVmax of baseline FDG PET/CT and treatment outcome after SBRT. There has been controversy whether the SUV of pretreatment FDG PET/CT determines prognosis [8]. Although there are reports that SUVmax is not helpful in predicting prognosis, recent meta-analyses [9,10] have recognized and bolstered the predictive value of SUVmax in FDG PET/CT. The fact that higher SUVmax is related to a less favorable prognosis has already been reported by Lee et al. [11], SUVmax 5 (the cutoff value used in the current study) is a baseline value reported in previous studies that appeared to determine the prognosis for distant metastasis [7] in SBRT.
In this study, we especially focused on the correlation between PFS and SUVmax values. In addition, we evaluated clinical factors, such as T stage or tumor size [12-15], which we expected to be related to prognosis. Local control (LC) and distant metastasis after treatment were also evaluated.
Materials and Methods
Twenty-seven patients who underwent baseline FDG PET/CT, among those who underwent SBRT treatment for primary lung cancer (cT1-3N0M0) at at Inje University Busan Paik Hospital, between January 2010 and May 2018, were analyzed. The Institutional Review Board of Busan Paik Hospital approved this study (No. 18-0199). Informed consent has been waived by the IRB because it was a retrospective chart review study. All patients underwent FDG PET/CT before SBRT to diagnose lung lesions. Additionally, all patients, except for one, underwent pathological diagnosis for the lung lesion before undergoing SBRT. TNM stage was designated according to the American Joint Committee on Cancer Staging 8th edition. N staging was based on pre-treatment PET/CT results. Mediastinal lymph node evaluation using endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy was not essentially conducted for this study. Tumors were divided into central and peripheral tumors according to the RTOG 0236 protocol [1]: central tumor was defined as a tumor located within 2 cm from the secondary branch of the proximal bronchus. Tumor response after SBRT was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1.
For SBRT, all patients were treated using the Varian RapidArc system (Varian Medical Systems Inc., Palo Alto, CA, USA). In the treatment plan, four-dimensional CT was performed to reflect movement by breathing. SBRT was performed in doses of ≥12 Gy per fraction. The total doses of 45 Gy, 48 Gy, 54 Gy, and 60 Gy were administered, divided into 3–5 fractions. The median total dose was 54 Gy. Biologically effective doses of all patients were >100 Gy10, which is known as the dose level for higher LC [16]. Large tumors (T2-3 stage) were generally irradiated with a small fraction size to reduce the pulmonary toxicity. Conversely, in the cases with tumor resection, the total radiation dose and fraction number were reduced. Most patients underwent first-line SBRT with the aim of curative local treatment. However, one patient (3.7%) was treated using SBRT for local recurrence after resection; other four (14.8%), who were treated with SBRT to address residual tumor after wedge resection, were also included.
After 6 hours of fasting, patients were injected with 4.44 MBq/kg of F-18 FDG for PET/CT scan. One hour after injection, patients underwent FDG PET/CT (Discovery STE; GE Healthcare, Milwaukee, WI, USA). In addition, the SUV was obtained and quantified using the AW VolumeShare 5 Workstation (GE Healthcare). FDG PET/CT parameters were collected and used for analysis. Patients were divided into two groups using a calculated cutoff SUVmax value.
Follow-up (FU) evaluation was performed by imaging test at 1 month, 3 months, and 6 months after SBRT, and additional outpatient clinic FU was performed more than once every 6 months thereafter. Local failure (progression) was defined as a least 20% of increase of diameter of target lesion. LC was measured in terms of time from the start of SBRT to local failure or last FU. PFS was measured on the basis of time from the first SBRT start date to any progression or last FU. Overall survival (OS) was defined as time from SBRT start to death or time to last FU. PFS was analyzed as a primary end-point. In addition, the overall progression rate, LC, and distant metastasis rate according to the FDG PET/CT results were also analyzed.
Statistical analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). The chi-squared test was initially used to determine prognostic factor(s) associated with progression. The Youden index was used to estimate the cutoff point in the receiver operating characteristic (ROC) curve (MedCalc software version 18.9; MedCalc, Mariakerke, Belgium). Kaplan-Meier analysis and log-rank test were used for survival analysis. Cox regression was used to determine an independent prognostic factor. A two-tailed p < 0.05 was considered to be statistically significant.
Results
1. Patients and treatment
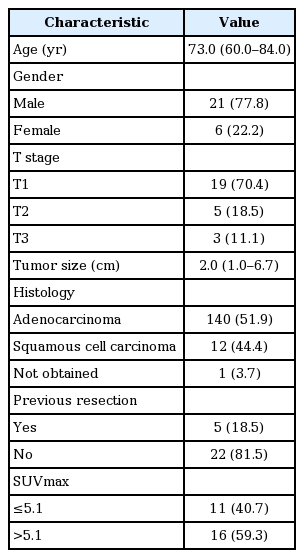
Patient characteristics are summarized in Table 1. The median age of the patients was 73.0 years (range, 60 to 84 years). All patients were >60 years of age, and males comprised 77.8% (n = 21) of the cohort. Slightly more than one-third of the patients (n = 11) did not undergo radical surgery before SBRT because of existing double primary cancer. With regard to other comorbidities, two patients had heart failure, two had obstructive lung disease, and one had a restrictive lung disease. Tumor was located in the right lobe in 20 patients (74.0%) and in the left lobe in 7 (25.9%). There were 20 (67.7%) cases of tumor located in the upper or middle lobe and 10 (33.3%) cases in the lower lobe. In this study, the all lung tumors belonged to the peripheral lesion (>2 cm from tracheo-proximal bronchial tree, 100%) based on RTOG 0236 protocol [1]. Staging was T1 in 19, T2 in 5, and T3 in 3. No patients had lymph node or distant metastasis at the start of SBRT. The median tumor size was 2.0 cm (range, 1 to 6.7 cm). In the baseline FDG PET/CT, the median SUVmax was 5.7 (range, 1.8 to 21.4). Depending on the pathological subtype, there were 14 cases of adenocarcinoma, 12 cases of squamous cell carcinoma, and one case in which a tissue sample was not obtained.
2. Patterns of failure
The median FU period of the patients was 17.7 months (range, 2.3 to 60.0 months). In addition, the median FU period for surviving patients was 15.9 months. The response rate was 33.3% (n = 9) and all responders exhibited partial response. Overall, 8 patients (29.6%) exhibited progression during the FU period. According to failure patterns, local failure occurred in 5 patients (18.5%) while regional lymph nodal failure occurred in 1 (3.7%). Distant metastases were observed in 2 (7.4%) cases. With regard to the metastatic sites, one patient exhibited brain metastasis and the other exhibited contralateral lung metastasis.
During the FU period, 7 patients (25.9%) died: 3 patients of lung cancer progression; 2 of heart failure; and the remaining 2 of intercurrent disease. Regarding treatment toxicity, 3 patients (11.1%) received oral steroids for radiation pneumonitis after treatment; however, all eventually recovered.
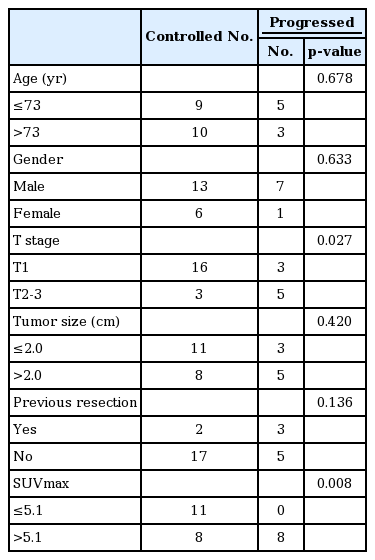
3. Factors related to progression
Using progression and SUVmax data, the optimal cutoff value was obtained using ROC curve analysis. SUVmax >5.1 in baseline FDG PET/CT (area under the ROC curve [AUC] = 0.786, p = 0.001, sensitivity 100%, specificity 57.9%) was calculated as the optimal cutoff point to predict patient progression (Fig. 1A). In the further subgroup analysis for patients without previous resection history (n = 22), the optimal cutoff point for progression was calculated as SUVmax >7.6 (AUC = 0.882, p < 0.001, sensitivity 100%, specificity 82.4%) (Fig. 1B).

Cutoff point for SUVmax in receiver operating characteristic curve for all patients (A) and for previously unresected patients (B). AUC, area under curve.
The occurrence of progression according to clinical factors are summarized in Table 2. SUVmax was significantly correlated with progression (p = 0.008). Patients with advanced T stage (i.e., T2-3) also exhibited higher overall progression (p = 0.027).
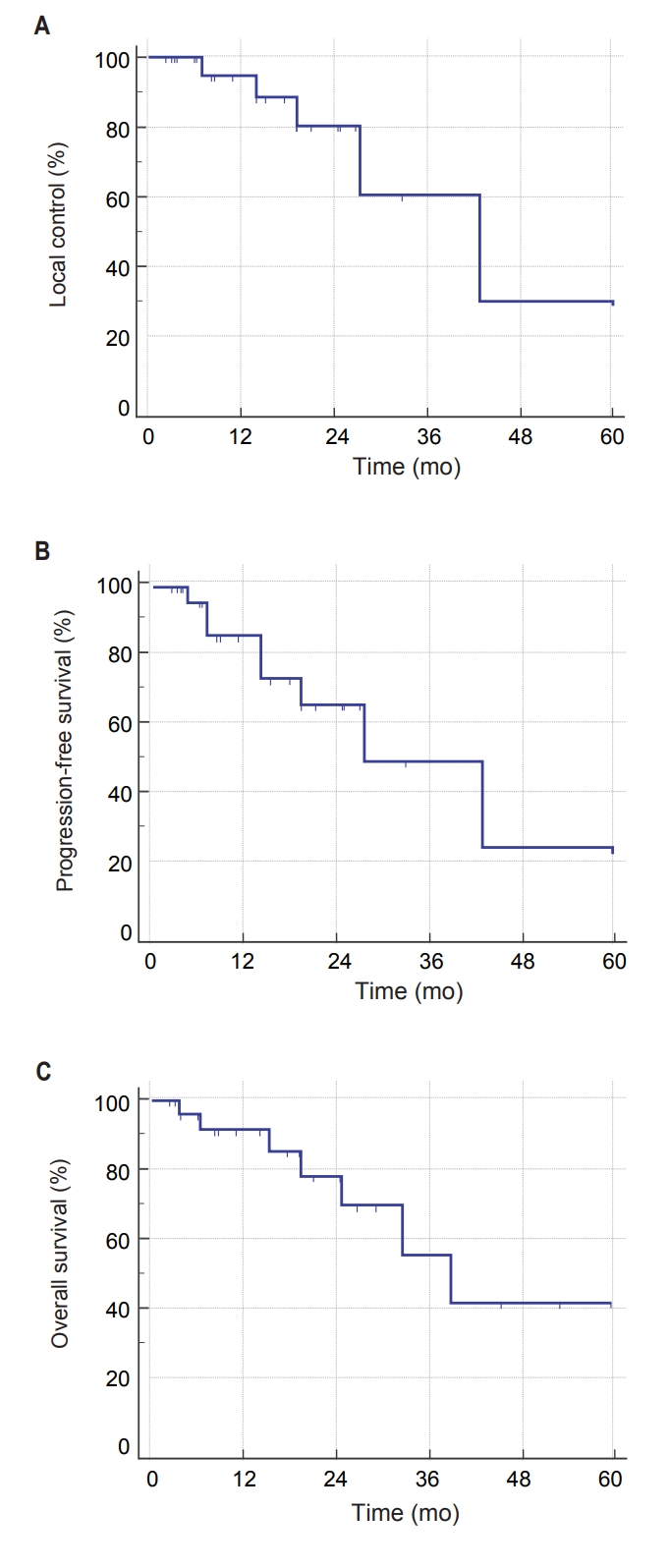
4. Prognostic factors for PFS
The actuarial 1-year LC, PFS, and OS were 94.7%, 85.6%, and 91.4%, respectively. Two-year LC was 80.4% and 2-year PFS was 66.0% (Fig. 2A, 2B). The 2-year OS was 78.2% (Fig. 2C).
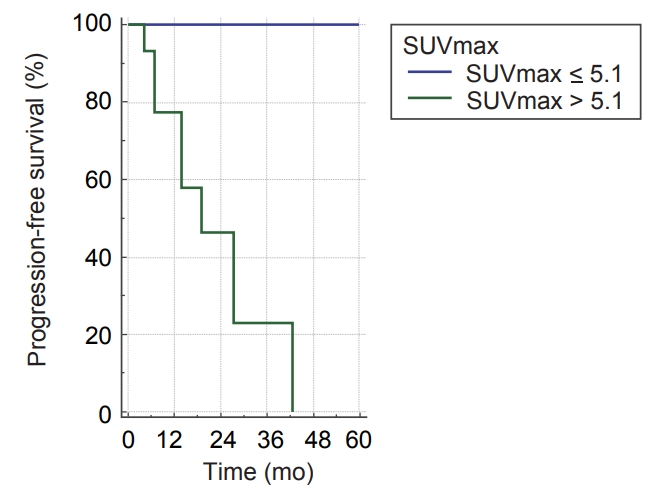
Differences in PFS according to clinical factors are shown in Table 3. In univariate analysis, PFS was significantly associated with T stage and SUVmax at baseline FDG PET/CT. PFS was significantly influenced by T stage (T1 vs. T2-3; p = 0.012) (Table 3) and pre-treatment SUVmax (SUVmax >5.1 vs. SUVmax ≤5.1; p = 0.012) (Fig. 3). Moreover, OS was not significantly affected by T stage (p = 0.993) or pre-treatment SUVmax (p = 0.716).
Discussion and Conclusion
The most important finding of this study was that pretreatment SUVmax was an important factor in determining prognosis of lung cancer. This study showed the influence of cellular metabolism on prognosis of lung cancer after SBRT. We suggested adequate cutoff points of the SUVmax value in predicting the prognosis of lung cancer. More specifically, a cutoff SUVmax value of 5.1 demonstrated a role in determining PFS. High FDG uptake of tumor is a reflection of aggressiveness. Patients with SUVmax >5.1 at baseline FDG PET/CT exhibited continuously decreasing in PFS over time. This indicates that there is a need for long-term close FU with the possibility of recurrence in these patients with higher SUVmax. On the other hand, compared with previous studies investigating SBRT [14,17,18], our study demonstrated that distant metastasis was not a dominant failure pattern. The lower distant metastasis rate of this study may be related with the short median follow-up period (17.7 months). Inversely, this study showed a higher local failure rate compared to the previous study by Baumann et al. [14]. To overcome the risk of local failure, additional local treatment could be considered (such as post-radiation resection or radiofrequency ablation [19]) in patients with large primary tumor size or higher SUVmax. Table 4 shows the detailed information about the progressed patients. The total radiation dose for half of resected lung cancer patients was relatively lower than other patients (Table 4). It should be noted that there were more recurrences in those patients treated with 45 Gy/3 fx SBRT after incomplete wedge resection (Table 4). According to this study, we should use full dose of radiation for postoperative SBRT.
Plus, the patient who underwent SBRT for local recurrence eventually relapsed again. In addition, one third of T3 patients (n = 1, tumor size = 6.7 cm) experienced local failure.
Based on the fact that pretreatment FDG PET/CT was helpful in determining the possibility of progression, it is reasonable to believe that serial FDG PET/CT (before and after SBRT) may be helpful in predicting patient outcome after treatment. Several studies have already reported that FDG PET/CT after SBRT was helpful in predicting prognosis [5,11,20,21]. In particular, FDG PET/CT can be useful to confirm local recurrence [6]. In this study, CT was performed mainly during patient FU because of the economic burden to the patient and the national health insurance policy of Korea. However, it is clear that posttreatment FDG PET/CT can be a good alternative diagnostic tool. If FDG PET/CT had a more reasonable cost, it could be applied during patient FU instead of CT. Conversely, the SUVmax of FDG PET/CT was not an independent prognostic factor according to the results of this study; thus, there is a limit to its diagnostic value. Therefore, efforts to develop a more sensitive diagnostic tool that can complement FDG PET/CT should be pursued.
Regarding limitations of this study, the number of patients in our institution was relatively small, which may have introduced selection bias. This study has limited statistical power because of small number of patients. Patients of SUVmax <5.1 showed no progressions in this study. However, it may reflect the bias from the small sample size. A multi-institutional study, therefore, could overcome this drawback. Additionally, because it was a retrospective study, the patient group was not homogeneous. Patients in the terminal stage are often referred to a nursing home or other hospital. For this reason, the accuracy of OS estimates tended to be low. Therefore, although this study demonstrated a fairly good OS, the results require careful, if not cautious, interpretation and application.
In conclusion, SUVmax at pre-treatment FDG PET/CT had a predictive value for PFS. FDG PET/CT can be a useful and credible modality for estimating prognosis in patients with primary lung cancer after SBRT.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.