Prognostic factors, failure patterns and survival analysis in patients with resectable oral squamous cell carcinoma of the tongue
Article information
Abstract
Purpose
There is sparse literature on treatment outcomes research on resectable oral tongue squamous cell carcinoma (OTSCC). The aim of this study was to measure the treatment outcomes, explore the failure patterns, and identify the potential clinicopathological prognostic factors affecting treatment outcomes for resectable OTSCC.
Materials and Methods
It is a retrospective analysis of 202 patients with resectable OTSCC who underwent upfront primary surgical resection followed by adjuvant radiotherapy with or without concurrent chemotherapy if indicated.
Results
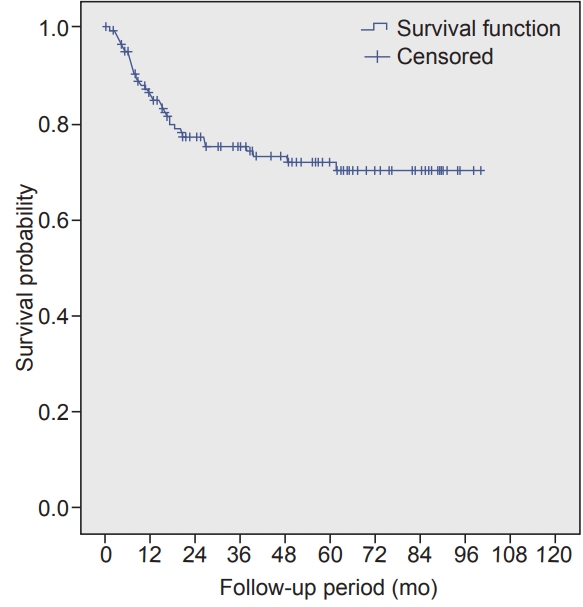
The median follow-up was 35.2 months (range, 1.2 to 99.9 months). The median duration of locoregional control (LRC) was 84.9 months (95% confidence interval, 67.3–102.4). The 3- and 5-year LRC rate was 68.5% and 58.3%, respectively. Multivariate analysis showed that increasing pT stage, increasing pN stage, and the presence of extracapsular extension (ECE) were significantly associated with poorer LRC. The median duration of overall survival (OS) was not reached at the time of analysis. The 3- and 5-year OS rate was 70.5% and 66.6%, respectively. Multivariate analysis showed that increasing pT stage and the presence of ECE were significantly associated with a poorer OS.
Conclusion
Locoregional failure remains the main cause of treatment failure in resectable OTSCC. There is scope to further improve prognosis considering modest LRC and OS. Pathological T-stage, N-stage, and ECE are strong prognostic factors. Further research is required to confirm whether adjuvant therapy adds to treatment outcomes in cases with lymphovascular invasion, perineural invasion, and depth of invasion, and help clinicians tailoring adjuvant therapy.
Introduction
Incidence of oral tongue squamous cell carcinoma (OTSCC) is on rise especially in the younger population [1-3], probably due to a rise in tobacco and alcohol intake. Early stage OTSCC is treated with single modality therapy preferably surgery [4], whereas locally advanced resectable disease is treated with combined modality therapy—surgery followed by adjuvant therapy with radiotherapy (RT) or chemoradiation (CRT). Although several clinicopathological prognostic factors have been found for oral cavity squamous cell carcinoma (OCSCC), most of these have been reported in studies done on mixed patient population with all subsites of oral cavity combined [5,6]. Data is limited for OTSCC per se. Since OTSCC is on rise and treatment outcome of OTSCC has been found to be poorer than that of carcinoma arising from other subsites of oral cavity [7,8], it is important to identify clinicopathological factors for carcinoma arising from this subsite. The aim of this study was to measure the treatment outcomes, explore the failure patterns, and identify the potential clinicopathological prognostic factors affecting treatment outcomes for resectable OTSCC.
Materials and Methods
The Institutional Ethics Committee of our institution approved the study. The inclusion criteria were patients with localized and resectable OTSCC with or without cervical nodal involvement who had undergone primary surgical resection of the primary tumor and regional lymph nodes at this institute between November 2010 and December 2016. The exclusion criteria included: an Eastern Cooperative Oncology Group performance status of ≥2; neoadjuvant chemotherapy administered prior to primary surgical resection; surgical resection performed with palliative or debulking intent; recurrent head and neck malignancies; and a prior history of RT to the head and neck region. Although the American Joint Committee on Cancer (AJCC) 7th edition was used for the staging and management, AJCC 8th edition was used to analyze prognostic factors in the analysis of this study. Adjuvant RT was administered within 4 to 6 weeks from the date of surgery depending on the high-risk features recorded in the histopathological report of the resected specimen. As per institutional policy, the following were the indications for adjuvant RT: pathological T3 or T4 stage, node positivity (even a single node), positive resection margins (<1.0 mm), close surgical margins (≥1.0 mm to ≤4.0 mm), perineural invasion (PNI), and lymphovascular invasion (LVI). Depth of invasion (DOI) of >4.0 mm was considered as an indication for adjuvant RT depending on the individual policy of treating physicians. Concurrent chemotherapy—weekly cisplatin (40.0 mg/m2) or carboplatin (area under the curve 2) was administered to patients with positive resection margins or extracapsular extension (ECE). Whilst planning adjuvant CRT, patients were initially considered for weekly cisplatin. Those who were elderly or presumed unfit for cisplatin were administered carboplatin or cetuximab.
Patients were treated with two-dimensional conventional RT or simultaneous-integrated boost intensity-modulated RT (SIB-IMRT). Conventional fractionation was used for all patients. For SIB-IMRT surgical bed with positive margin or nodal region with ECE were given 66 Gy. Surgical bed without positive margin or nodal regions without ECE were given 60 Gy in 30 fractions. Elective nodal regions were given 54 Gy in 30 fractions. When two-dimensional parallel opposed shrinking field technique was used we delivered 50 Gy in 25 fractions to elective nodal regions and boosted surgical bed without margin positive or nodal regions without ECE to 10 Gy in 5 fractions. Surgical bed with positive margin or nodal region with ECE was boosted to another 6 Gy in 3 fractions.
1. Statistical analysis
All statistical analyses were performed using statistical package for the social science system (SPSS version 20; IBM SPSS, Armonk, NY, USA), and p-value less than 0.05 was considered statistically significant. All p-values reported represent two-sided tests. Baseline clinical and pathological categorical variables were expressed as frequencies along with respective percentages. The endpoints were locoregional control (LRC) and overall survival (OS), and were calculated by the Kaplan–Meier product-limit method. Univariate analysis of LRC and OS was performed on the following clinical and histopathological factors selected based on results from previous studies on oral cavity cancer: age, sex, addictions (tobacco smoking, tobacco chewing and/or alcohol consumption), tumor grade, pathological T (pT) stage, pathological N (pN) stage, PNI, LVI, resection margin status, DOI, and ECE. Multivariate Cox proportional hazards regression analysis was performed to estimate the impact of known relevant prognostic factors.
Results
The medical records of 202 patients fulfilling inclusion and exclusion criteria were reviewed. Patient and disease-related characteristics are shown in Table 1. The median follow-up was 35.2 months (range, 1.2 to 99.9 months).
The median duration of LRC was 84.9 months (95% confidence interval, 67.3–102.4). The 3- and 5-year LRC rate was 68.5% and 58.5%, respectively (Fig. 1). Univariate analysis showed that increasing pT stage, increasing pN stage, and the presence of PNI, LVI, and ECE were significant poor prognostic factors for LRC. However, multivariate analysis showed that increasing pT stage, increasing pN stage, and the presence of ECE were significantly associated with poorer LRC (Table 2).
The median duration of OS was not reached at the time of analysis. The 3- and 5-year OS rate was 70.5% and 66.6, respectively (Fig. 2). Univariate analysis revealed that increasing pT stage, increasing pN stage, presence of LVI, DOI of >20 mm, and ECE were poor prognostic factors for OS. However, multivariate analysis using Cox proportional hazard ratios showed that increasing pT stage and the presence of ECE were significantly associated with a poorer OS (Table 3).
Majority of percent patients completed planned treatment. At the time of analysis, 74 patients (36.6%) had developed recurrence. Of these, 33 patients (44.5%) experienced tumor recurrence in the primary site alone, 23 (31.1%) experienced recurrence in the nodal region alone, 3 (4.0%) experienced recurrence in the primary site and nodal region, and 15 (20.2%) had distant metastases most common being lung followed by bone.
Discussion and Conclusion
This study is an attempt to determine LRC and OS, failure pattern and explore various prognostic clinical and pathological factors influencing LRC and OS for OTSCC. This study continued to provide yet another evidence that locoregional recurrence remains a major mode of treatment failure in this subset of patients, considering approximately a third of recurrence being locoregional in this study. Hence, thorough attempt should be made to identify high-risk patients for recurrence based on clinical and pathological factors for whom additional therapy in the form of adjuvant RT or CRT may improve outcomes.
Although there were 38.6% patients in the current study who were found to have pathological stage III/IV, more than two-thirds of patients from the entire cohort received adjuvant RT because of combination of other risk factors on surgical histopathological specimen examination. Approximately one-fourth amongst those who received adjuvant RT also received concurrent chemotherapy (CCT).
Gender may have an influence on the treatment outcome as shown by various studies with large sample population including all subsites of oral cavity combined together [9-12]. These studies showed female gender being associated with better survival. The current study did not show any association with outcomes in line with Garavello et al. [13] who particularly looked at the influence of gender on OTSCC similar to our study, and found that gender does not influence prognosis in this subsite. Age whether as continuous or categorical variable was not found to affect outcome in OTSCC in this study. However in a Surveillance, Epidemiology and End Results database analysis, there was found to be a significant difference in median OS between age groups: 50–69 years and ≥70 years with OTSCC (59.5 vs. 73.1 months, respectively); however the difference nullified when results were analysed after stage stratification [14]. Elderly patients tend to have multiple comorbidities which may influence the choice of therapy, intent of therapy, course of intensive treatment such as major surgeries or adjuvant CRT and may have poor tolerance to treatment, thereby showing different response to therapy. Thus, it appears that association of prognosis with age is complex and less clearly understood as yet in OTSCC.
A number of reports have showed that alcohol consumption and smoking are prognostic factor for head and neck cancer [15-18]. When looking at OTSCC per se, Sawabe et al. [19] in a prospective study showed that patients with OTSCC treated with surgery is associated with a significant inverse association between alcohol consumption and prognosis. However we did not find these habits be a prognostic factor in Indian population particularly looking at OTSCC. Our results are similar to that of a study done by Thiagarajan et al. [20] on similar cohort of patients as ours (Indian patients with OTSCC), that habits (including alcohol, smoking and tobacco chewing) does not affect the OS.
In the OTSCC, T-stage and N-stage are independent indicators of the prognosis, although they are inter-related. Increasing size by T-stage leads to an increase in the rate of occult metastases [21-23], and increasing N-stage is associated with the development of distant metastases, particularly with multiple involvement or the presence of ECE [22]. Increasing pT stage emerged from our study as being of high prognostic value, as the risk of local recurrence at the time of diagnosis increased when tumour size increases from less than 2 cm to more than 4 cm. The benefit in OS increased as the pT stage increased from T2 to T4. This experience is similar to results found in other reports [24].
Presence of cervical lymphadenopathy is shown as single most important clinical predictor of OS in this study. However, mere presence of single node less than 3 cm did not affect OS, it was the presence of multiple nodes which affected OS significantly poorly. There were no patients with clinical N3 stage because surgeons found them to be unresectable, and hence were given radical RT/CRT. There were no patients who were upstaged to pN3 stage either. All patients received CCT in this study if there were ECE. This was based on the combined analysis of landmarks trials EORTC-22931 and RTOG-9501 studies which showed advantage of adding CCT [25]. Despite adding CCT in the presence of ECE in our study patients, it still continued to show significant impact on OS on multivariate analysis. When looking particularly at OTSCC high quality data is sparse regarding the impact of ECE. Our results are consistent with few retrospective studies which showed ECE as a significant predictor of OS in OTSCC [20,26,27].
DOI is used as a surrogate for risk of lymphatic involvement. Hence, neck dissection (elective neck dissection) has become an acceptable neck management strategy for OTSCC when the DOI is high [28,29]. Most centers regard 3.0 to 4.0 mm as the optimal cutoff for DOI, beyond which neck management is essential for the clinically node-negative neck. There has been considerable controversy regarding the need for adjuvant RT in patients without high-risk features, except for a DOI of >4.0 mm. Following the results published by Ganly et al. [30], from the Memorial Sloan Kettering Cancer Center and Princess Margaret Hospital in 2013, which demonstrated much higher regional failure than anticipated in patients with pT1/2 and pN0 OSCC, who underwent partial glossectomy and ipsilateral elective neck dissection without postoperative RT, our institution has accepted a DOI of >4.0 mm alone as a sufficient risk factor to offer adjuvant RT in the majority of these cases, although it influenced OS in this study in univariate analysis only when it was >20 mm. Few other studies have used multivariate analysis to substantiate the importance of the DOI in OTSCC or have revealed a correlation between the DOI and LRC or disease-free survival [31-33]. A recent study by Gokavarapu et al. [34] showed that postoperative RT did not influence LRC or OS in a subset of patients with pT1/2 and pN0 OSCC with a DOI of ≥4.0 mm. Although it appears certain that DOI has prognostic role for OCSCC, it is yet to be evaluated beyond which DOI in mm adjuvant RT benefit significantly.
LVI has been shown to be significantly associated with tumor site, diameter, and thickness, PNI, the invasive front, the pattern of invasion, lymph node metastasis, resection margin status, local recurrence, and survival [22,35-37]. Consistent with these findings, our study showed a significant association between LVI and OS in the univariate analysis. However, in the multivariate analysis, LVI was not found to be an independent predictor of OS. A review of the literature identified only one study that reported LVI to be significant in the multivariate analysis. Rahima et al. [38] discussed the possibility of using this parameter as a criterion to define aggressiveness and to select patients for more specific and aggressive treatment in the future.
In the analysis by El-Husseiny et al. [39] on OTSCC (T1-4 N0-3) the reported 5-year OS was 65%. Rusthoven et al. [7] described a 5-year OS of 60.9% in patients treated for T1-2 N0 OTSCC. Although the prognosis of OTSCC was poorer than that of cancers in other oral cavity sites, in this study, the 5-year OS and LRC were shown as 66.6% and 58.3%, respectively. This study showed the survival outcome of OTSCC to be affected by increasing T stage, more than two clinically positive nodes, ECE of lymph node metastasis and LVI on univariate analysis. These findings have also been reported by others [24,40-42]. However, multivariate analysis revealed increasing pT stage and presence of ECE to be significantly associated with overall survival.
The limitations of this study include potential bias associated with its retrospective design. Although the treatment guidelines followed at our institute are standardized, some differences remain in the indications of adjuvant therapy in OCSCC depending on individual experiences of the treating oncologist, especially with regards to DOI. A strength of this study is its large cohort of patients with squamous cell carcinoma limited to oral tongue subsite only, taken from one of the largest and most exclusive cancer research centers in the country. Furthermore, the fact that all surgical and histopathological reporting was done at a single institute may have helped to reduce bias.
In conclusion, locoregional failure remains the main cause of treatment failure in resectable OTSCC. There is scope to further improve prognosis considering modest LRC and OS. Pathological T-stage, N-stage, and ECE are strong prognostic factors. Further research is required to confirm whether adjuvant therapy adds to treatment outcomes in cases with LVI, PNI and DOI, and help clinicians tailoring adjuvant therapy.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.