Local ablative radiotherapy for oligometastatic non-small cell lung cancer
Article information
Abstract
In metastatic non-small cell lung cancer (NSCLC), the role of radiotherapy (RT) has been limited to palliation to alleviate the symptoms. However, with the development of advanced RT techniques, recent advances in immuno-oncology therapy targeting programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) and targeted agents for epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation allowed new roles of RT in these patients. Within this metastatic population, there is a subset of patients with a limited number of sites of metastatic disease, termed as oligometastasis that can achieve long-term survival from aggressive local management. There is no consensus on the definition of oligometastasis; however, most clinical trials define oligometastasis as having 3 to 5 metastatic lesions. Recent phase II randomized clinical trials have shown that ablative RT, including stereotactic ablative body radiotherapy (SABR) and hypofractionated RT, to primary and metastatic sites improved progression-free survival (PFS) and overall survival (OS) in patients with oligometastatic NSCLC. The PEMBRO-RT study, a randomized phase II study comparing SABR prior to pembrolizumab therapy and pembrolizumab therapy alone, revealed that the addition of SABR improved the overall response, PFS, and OS in patients with advanced NSCLC. The efficacy of RT in oligometastatic lung cancer has only been studied in phase II studies; therefore, large-scale phase III studies are needed to confirm the benefit of local ablative RT in patients with oligometastatic NSCLC. Local intensified RT to primary and metastatic lesions is expected to become an important treatment paradigm in the near future in patients with metastatic lung cancer.
Introduction
Globally, lung cancer accounts for 12% (2.1 million) of new cancer cases and 18% (1.8 million) of cancer-related deaths based on the global cancer statistics of 2018 [1]. In both genders, lung cancer is the most commonly diagnosed cancer and the most common cause of cancer death. In Korea, lung cancer was the fourth most commonly diagnosed cancer (26,000) and the leading cause (18,000) of cancer death in 2016 [2]. The 5-year relative survival rate of lung cancer between 2012 and 2016 was 28% in Korea. Platinum-based doublet chemotherapy has been the standard treatment for metastatic non-small cell lung cancer (NSCLC), but it leads to poor outcomes with a median survival of 8–11 months [3,4]. Thus, the role of radiotherapy for advanced lung cancer has been limited to palliative treatment to alleviate patients’ symptoms. However, the development of advanced radiotherapy techniques and recent advances in pharmacotherapy, including cytotoxic chemotherapy, molecular-targeted therapy, and immune checkpoint blockade, have resulted in a growing interest for local ablative therapy (LAT) for oligometastatic diseases. Currently, there is no consensus on the definition of oligometastasis. However, most studies defined oligometastatic disease as having 1–3 or 1–5 metastatic lesions [5-8]. In this review, we will briefly describe the biological and clinical rationales of LAT for oligometastasis and summarize the recent prospective clinical trials on LAT for oligometastatic NSCLC.
Biological Rationale of LAT for Oligometastasis
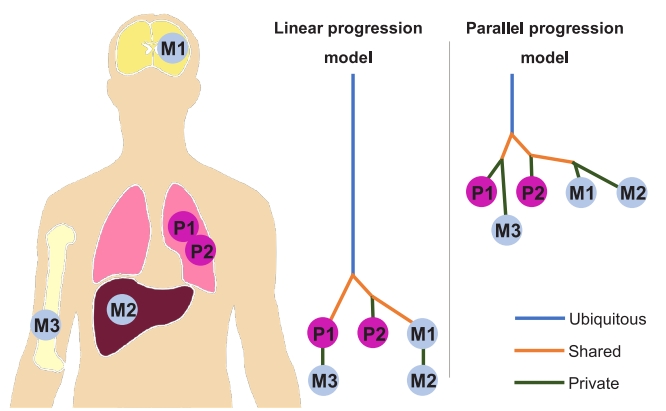
Recent next-generation sequencing (NGS) technology allows the phylogenetic analysis of primary and metastatic tumors and reveals branched evolutionary tumor progression [9-11]. Genomic analysis in patients with NSCLC has shown that primary tumors and lung metastasis share a common driver mutation [12,13]. These data indicate that primary tumor and its metastases are clonally related, and they originate from a common ancestral cell. Phylogenetic trees or relationships of primary and metastatic subclones can be estimated by analysis of ubiquitous, shared, and private mutations. According to the relative timing of the emergence of new metastatic subclones and degree of genetic divergence between primary and metastatic tumors, models of metastatic evolution or cascade can be classified into linear and parallel progression models [14-16]. Early emergence of metastatic subclones and high degree of genetic divergence favor the parallel progression model of metastatic cascade, while late emergence of metastatic subclones and low degree of genetic divergence support the linear progression model [16,17] (Fig. 1). In the linear progression model, metastasis can occur late during disease progression; however, in the parallel progression model, early dissemination of tumor cells can occur. In both linear and parallel progression models, intratumoral heterogeneity of the primary tumor and earlier metastasis can lead to further disseminated metastases. As a result, LAT to the primary tumor and early metastatic lesions may prevent emergence of new metastasis-competent clone and progression to disseminated metastasis. Therefore, improving survival by performing LAT at the limited metastatic stage is possible in the two progression models of metastatic evolution.
Radiotherapy Techniques
LAT options for patients with limited metastasis include surgical resection, radiotherapy, radiofrequency ablation, and cryoablation [18-22]. Radiotherapy is a nonsurgical and noninvasive treatment, and high-dose radiation shows good tumor control. Stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiotherapy, refers to stereotactically guided delivery of highly conformal radiation with ablative dose in a limited number of fractions [23]. SABR for metastatic lesions in various sites, including the lung, liver, adrenal gland, lymph nodes, and spine, has shown good local control rates ranging from 70% to 100% and acceptable treatment-related toxicities [24-30]. Typically, noninvasive positioning techniques, such as those in imageguided radiotherapy, and short treatment courses with 1, 3, or 5 fractions are used. The ongoing randomized phase II/III trial (NRG-LU002) comparing local consolidative therapy and maintenance systemic therapy for limited metastatic NSCLC uses radiation doses of 24 Gy (acceptable variation, 21–27 Gy) with 1 fraction, 30 Gy with 3 fractions (acceptable variation, 26.5–33 Gy), and 34 Gy with 5 fractions (acceptable variation, 30–37.5 Gy) for SABR. For the primary site, 45 Gy (acceptable variation, 42–48 Gy) with 15 fractions is used.
Interestingly, a recent systematic study analyzed the effects of the types of LAT on OS in oligometastatic NSCLC patients [31]. According to this study, the use of SABR instead of conventionally fractionated radiotherapy for these patients rapidly increased after 2011, and a time trend towards improved OS after 2011 was detected. Moreover, the types of LAT including radiotherapy and surgery for primary tumor or distant metastasis did not result in a significant effect on median OS.
1. Radiotherapy for oligometastatic NSCLC
The Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastasis (SABR-COMET) study, a randomized phase II trial, demonstrated that the addition of SABR to all sites of disease improved the overall survival (OS) and progression-free survival (PFS) in patients with controlled primary tumor and up to 5 metastatic lesions [32]. In this study, 99 patients with breast, prostate, and lung cancers were randomized to receive standard of care with palliative treatments (control group) or standard of care with SABR to all sites of metastatic disease (SABR group) in a 1:2 ratio. The use of SABR resulted in a 13-month improvement in the OS (median OS, 28 months vs. 41 months) with a doubling of PFS (median PFS, 6 months vs. 12 months). The frequencies of adverse events ≥grade 2 were 9% and 29% in the control and SABR groups, respectively. Another randomized phase II trial also showed the survival benefit of local radiotherapy for stage IV NSCLC with ≤3 metastatic lesions [7]. After first-line systemic therapy, 49 patients without disease progression were randomly assigned to receive local consolidative therapy to all known disease sites including primary and lymph nodes or maintenance treatment in a 1:1 ratio. Local consolidate therapy consisted of SABR, intermediately hypofractionated radiotherapy (15 fractions to the mediastinum), and concurrent chemoradiotherapy, and surgery to all sites or combined with radiotherapy. The long-term results of this study also confirmed that local consolidative therapy improved the OS (median OS, 17 months vs. 41 months) and PFS (median PFS, 6 months vs. 12 months) [33].
2. Radiotherapy for patients with epidermal growth factor receptor mutation
Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) treatment improves OS in advanced NSCLC with EGFR mutation compared with standard first-line systemic treatment with a platinum-based doublet regimen [4]. This landmark study has changed clinical practices in patients with advanced NSCLC. Presently, molecular biology tests are essential in determining a treatment strategy, and EGFR-TKI is the first-line treatment for patients with activating mutations [34]. However, drug resistance and disease progression are inevitable during first- and second-generation EGFR-TKI treatments. Several phase III studies have shown a median PFS of 9–13 months with EGFR-TKI treatments [35-37]. Although third-generation EGFR-TKI targeting the EGFR p.Thr790Met point mutation (T790M) shows superior PFS than standard EGFR-TKIs only targeting exon 19 deletion and exon 21 L858R point mutation [38], drug-resistant mutations eventually develop in approximately 40% of patients [39,40]. When acquired resistance developed, local progression occurred in approximately one-fourth of patients, and LAT was suitable in approximately half of patients [41]. Therefore, LAT can be reasonable for patients with oligoprogression during EGFR-TKI treatment.
Xu et al. [42] retrospectively analyzed the survival benefit of LAT in patients with stage IV EGFR-mutant NSCLC who have oligometastatic disease with ≤5 metastatic lesions. In this study, after first-line EGFR TKI treatment, 51 patients received LAT for both primary tumor and all metastatic sites (all-LAT group), 55 patients received LAT for either primary tumor or metastatic sites (part-LAT group), and 39 patients did not receive LAT (non-LAT group). The all-LAT group shows significantly better PFS than the part-LAT group (hazard ratio [HR] = 0.47) and non-LAT group (HR = 0.32). The median OS of the all-LAT group (41 months) was also better than those in the part-LAT group (34 months) and non-LAT group (HR = 0.32). However, between the part-LAT and non-LAT groups, differences in the PFS and OS were not significant. In the multivariate analysis, exon 19 deletion (vs. exon 21 L858R point mutation), single metastasis (vs. multiple metastasis), and LAT for primary tumor (vs. no LAT for primary tumor) were associated with better OS. These results indicate that LAT for all metastatic sites as well as primary tumor may be paramount in improving OS.
A single-arm phase II study (NCT01941654) investigated the effects of preemptive LAT to residual oligometastasis (ATOM) in patients with advanced NSCLC with exon 19 deletion or L858R point mutation. After 3 months of first-line EGFR-TKI treatment, when ≤4 positron emission tomography-avid lesions persisted, LAT by either SABR or surgery was performed and EGFR-TKI treatment was continued after LAT. In this prospective study, 18 patients were enrolled, and 16 patients showed promising PFS (1-year PFS rate post-LAT, 63%).
These data indicate that LAT can be an attractive option for patients with oligopersistent or oligoprogressive disease during EGFR-TKI treatment. Pan-Asian-adapted clinical practice guidelines recommend LAT in patients with oligoprogression after first-line EGFR-TKI treatment [34].
3. Radiotherapy with immune checkpoint blockade
In patients with lung adenocarcinoma, approximately one-fifth to one-third patients have EGFR mutation, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (ALK) fusion, or c-ros oncogene 1 fusion [43-45]. They are good candidates for targeted therapy, including EGFR-TKI and ALK inhibitor treatment. However, in patients with lung squamous cell carcinoma, the rate of EGFR mutation is extremely low [46,47]. Therefore, treatment options for patients with lung squamous cell carcinoma are limited to cytotoxic agents.
Costimulatory molecules of T cells, including programmed death ligand 1 (PD-L1), programmed death 1 (PD-1), and cytotoxic T-lymphocyte-associated antigen 4, induce T-cell exhaustion and contribute to the escape of tumor cells from host immune surveillance [48]. Recent clinical trials have demonstrated that compared with conventional cytotoxic chemotherapy in patients with advanced or previously treated NSCLC, including squamous cell carcinoma, immune checkpoint blockades inhibiting suppressive costimulatory molecules of T cells improved OS [49-53]. The randomized phase III PACIFIC trial showed that compared to placebo therapy, adjuvant durvalumab treatment blocking the interaction of PD-1 with PD-L1 after concurrent chemoradiation for stage 3 NSCLC improved OS [54]. As a result, the role of radiotherapy in immuno-oncology has emerged.
A recent single-arm phase II study evaluated LAT combined with pembrolizumab treatment targeting PD-1 in patients with NSCLC with ≤4 metastatic lesions [55]. After performing LAT to all known sites of disease, pembrolizumab was administered for 6 months and continued to 12 months in the absence of disease progression or untoward toxic effects. A combination of LAT and pembrolizumab treatment resulted in promising outcomes with a 2-year OS rate of 77.5% and a median PFS of 18.7 months. A randomized phase II PEMBRO-RT trial also showed the benefit of SABR combined with pembrolizumab treatment [56]. Previously treated patients with NSCLC were randomly assigned to SABR plus pembrolizumab arm or pembrolizumab alone arm. SABR, which consisted of 24 Gy in 3 fractions, was performed in a single tumor site within 7 days prior to the first cycle of pembrolizumab treatment. SABR prior to pembrolizumab treatment significantly improved the overall response rate compared with pembrolizumab treatment alone (36% vs. 18%). The addition of SABR showed a tendency towards better PFS and OS than pembrolizumab treatment alone, although these results did not reach statistical significance. Interestingly, in the subgroup analyses, the largest benefit from the addition of SABR was observed in patients with PD-L1-negative tumors.
Ongoing Clinical Trials
Although several prospective trials have demonstrated the survival benefit of ablative radiotherapy for oligometastatic NSCLC, these were phase II studies and enrolled a small number of patients (Table 1). Presently, several ongoing phase III trials analyze the effects of ablative radiotherapy for oligometastatic NSCLC. The phase II/III trial in the UK (NCT02759783) compares conventional care versus radioablation for extracranial oligometastasis (CORE) in patients with prostate cancer, breast cancer, and NSCLC. The SARON trial (NCT02417662) is a randomized and multicenter phase III trial that is assessing the addition of SABR and radical radiotherapy to standard chemotherapy for oligometastatic NSCLC [57]. The SABR-COMET 3 (NCT03862911) is a phase III randomized trial and will evaluate the survival benefit of the addition of SABR to standard systemic treatments in oligometastatic cancer with 1–3 metastatic lesions. The SABR-COMET 10 phase III randomized trial (NCT03721341) also assesses the addition of SABR in patients with 1–10 metastatic lesions. The NRG-LU002 trial is a phase II/III trial that evaluates the addition of SABR or hypofractionated radiotherapy for all disease sites to standard systemic therapy. These phase III trials are expected to confirm the role of SABR or ablative radiotherapy in oligometastatic NSCLC.
Future Perspectives
Despite the abundant clinical and biological evidences that support the need for LAT for oligometastatic diseases, there are inadequate relevant biological markers predicting the prognosis of oligometastatic diseases and treatment response of LAT. To maximize the benefit of LAT, novel biomarkers for proper patient selection should be defined. Moreover, with the advances of immune checkpoint blockades and targeted agents, the optimum combination of LAT and these drugs should be investigated. Additionally, for better LAT, a comparison of SABR and minimally invasive surgery in future prospective trials is needed.
Conclusion
Recent phase II trials have shown that LAT in patients with lung cancer and limited metastatic diseases improved PFS and OS. The combination of SABR to metastatic lesions and hypofractionated radiotherapy to the primary site with standard systemic treatments seems to produce paramount treatment outcomes. Moreover, LAT demonstrated reasonable outcomes when combined with EGFR-TKI or immune checkpoint inhibitor treatment. With the development of novel biomarkers, targeted therapies, and immuno-oncologic agents, LAT, including SABR and ablative hypofractionated radiotherapy, will be an attractive new treatment strategy for oligometastatic NSCLC in the near future.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.