Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes
Article information
Abstract
Purpose
Mefenamic acid (MEF) as a non-steroidal anti-inflammatory drug is used as a medication for relieving of pain and inflammation. Radiation-induced inflammation process is involved in DNA damage and cell death. In this study, the radioprotective effect of MEF was investigated against genotoxicity induced by ionizing radiation in human blood lymphocytes.
Materials and Methods
Peripheral blood samples were collected from human volunteers and incubated with MEF at different concentrations (5, 10, 50, or 100 µM) for two hours. The whole blood was exposed to ionizing radiation at a dose 1.5 Gy. Lymphocytes were cultured with mitogenic stimulation to determine the micronuclei in cytokinesis blocked binucleated lymphocyte.
Results
A significant decreasing in the frequency of micronuclei was observed in human lymphocytes irradiated with MEF as compared to irradiated lymphocytes without MEF. The maximum decreasing in frequency of micronuclei was observed at 100 µM of MEF (38% decrease), providing maximal protection against ionizing radiation.
Conclusion
The radioprotective effect of MEF is probably related to anti-inflammatory property of MEF on human lymphocytes.
Introduction
Exposure to ionizing radiation (IR) is associated with increased DNA damage and leads to stress response and inflammation, even carcinogenesis and cell death. IR produces free radicals and other reactive substance and induces reactive oxygen species (ROS) which is main mechanism involved in DNA damage. ROS interacts with critical macromolecules such as DNA and oxidizes DNA [12]. There is a cross talk between ROS mediated inflammation and DNA damage. IR modulates several signaling pathways resulting in transcription factor activation. In response to DNA damage, IR activates inflammatory process through expression of proinflammatory cytokines such as interleukin-1 and 6 (IL-1 and IL-6) and mediators of cytokines such as nuclear factor-kappa B and tumor necrosis factor-alpha (TNF-α) [34]. Theses cytokines has an anti-apoptotic effect and promotes cell proliferation [56]. Several studies demonstrated that antioxidants scavenged free radicals in the irradiated cells and resulted in decreased inflammation process [789].
Mefenamic acid (MEF) is a non-steroidal anti-inflammatory drug (NSAID) relieving dental and menstrual pains. It is typically prescribed for oral administration. MEF provided significant protection against the elevation of lipid peroxidation, protein oxidation, levels of TNF-α and IL-1β and finally decreased inflammation in the brain of rat [1011]. In vitro , MEF could also reduce ROS production, lipid peroxidation, and protein oxidation [1213]. IR induced genotoxicity is wellknown to increase the risk of developing malignancy and to be linked to IR induced inflammatory processes suggesting that an anti-inflammatory drug may protect IR induced DNA damages. The effect of anti-inflammatory MEF has not been clearly revealed yet on irradiated normal human cells. Thus we investigated the IR induced genotoxicity on human normal lymphocytes priming with or without MEF.
Materials and Methods
1. Blood treatment
After obtaining permission from research and ethical committees of the Mazandaran University of Medical Sciences, this study was performed. Healthy, non-smoking three male volunteers, aged from 20 to 28 years were enrolled in this study. Twelve milliliters of whole blood were collected in heparinized tubes and divided in centrifuge tubes at 0.9 mL each. Blood samples were primed with 100 µL solution of MEF (Alhavi Pharmaceutical Company, Tehran, Iran) at the concentrations of 5, 10, 50, or 100 µM. These samples were incubated for two hours at 37℃. MEF was dissolved in dimethyl sulfoxide (DMSO; Merck Company, Darmstadt, Germany) and diluted in RPMI cultural medium (Gibco, Carlsbad, CA, USA). DMSO concentration was same in control and MEF solutions (0.2%).
2. Ionizing radiation and micronucleus test
Whole blood samples in tubes were irradiated with 6 MV X-ray beam produced by a linear accelerator (Primus; Siemens, Erlangen, Germany) at a dose of 1.5 Gy with a dose rate of 1.9 Gy/min. Samples from three volunteers were allocated to the control (non-irradiated samples). After irradiation, subsequently, 0.5 mL of each sample (control and irradiated samples in duplicate) was added to 4.4 mL of RPMI 1640 culture medium (Gibco), which contained a mixture of 10% fetal calf serum, 100 µL phytohemagglutinin (Gibco). All cultures were incubated at 37℃ in a humidified atmosphere of 5% CO2 and 95% air. Cytochalasin B (final concentration, 6 µL/mL; Sigma-Aldrich, St. Louis, MO, USA) was added after 44 hours of culture. Following 72 hours of incubation, the cells were collected by centrifugation, re-suspended in cold 0.75 M potassium chloride. Cells were immediately fixed in a fixative solution as made of methanol: acetic acid (6:1) two times. The fixed cells were dropped onto clean microscopic slides, air-dried and stained with 10% Giemsa (Merck) solution. All slides were evaluated at 100× magnification in order to determine the frequency of micronuclei in the cytokinesisblocked binucleated cells with a well-preserved cytoplasm [14]. For each treated group from each volunteer, a total of 1,000 binucleate cells (in the duplicate cultures) were examined to record the frequency of micronuclei-containing cells. A total of 3,000 binucleated lymphocytes were counted in each treated group from three volunteers, and totally 30,000 binucleated lymphocytes were counted for ten treated groups in this study.
3. Statistical analysis
For each volunteer, the frequency of IR-induced micronuclei was determined at each concentration of MEF. The data were analyzed with Student t-test. A probability value of 0.05 was accepted to denote significance.
Results
A typical binucleated lymphocyte with micronucleus is shown in Fig. 1. The mean frequency of micronuclei in irradiated three volunteers was 9.23 ± 0.63, while that in non-irradiated control samples was 0.63 ± 0.21. It showed a statistically significant (p < 0.001) increase (14-fold rise) in the frequency of micronuclei in irradiated samples at dose of 1.5 Gy (Table 1). In irradiated samples with MEF, the frequency of micronuclei at the concentrations of 5, 10, 50, or 100 µM were 9.10 ± 0.71, 7.80 ± 0.40, 6.10 ± 0.62, and 5.73 ± 0.51, respectively (Fig. 2 and Table 1). The data demonstrate that irradiated samples with MEF at concentrations of 10, 50, and 100 µM exhibited a significant decrease in the frequency of micronuclei as compared to irradiated samples without MEF. Total micronuclei frequencies were reduced by 1.4%, 15%, 34%, and 38% in irradiated samples with MEF at concentrations of 5, 10, 50, or 100 µM, respectively, compared to irradiated samples without MEF (Table 1). Among irradiated samples with MEF, a statistically significant difference in micronuclei was observed between doses 10 and 50 µM of MEF. It is interesting, the frequency of micronuclei in non-irradiated sample with MEF at concentration of 10 and 100 µM was significantly lower than control group (p < 0.05).

The frequency of micronuclei induced in vitro by 1.5 Gy X-ray radiation (IR) in cultured blood lymphocytes at different doses of mefenamic acida)
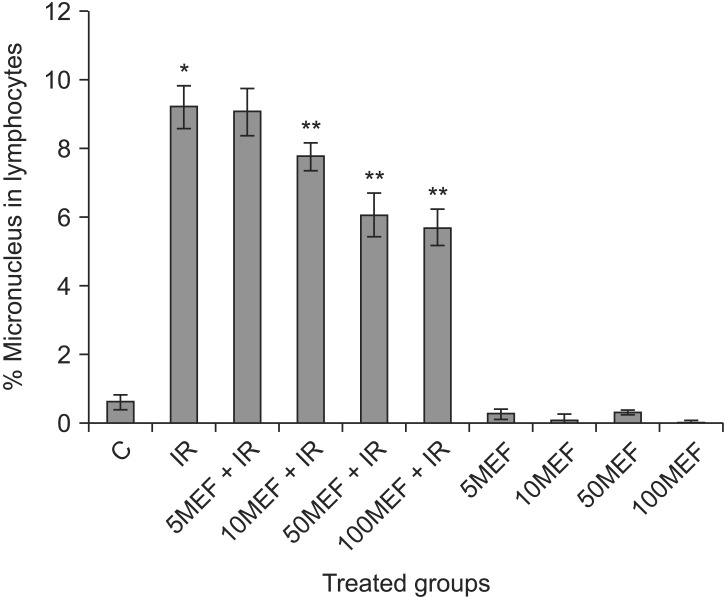
In vitro protection by mefenamic acid (MEF) at different concentrations (5, 10, 50, and 100 µM) against ionizing radiation (IR) induced genetic damage in cultured whole blood lymphocytes. The data represent average ± standard deviation from three volunteers. C, control; 5MEF, 5 µM mefenamic acid; 10MEF, 10 µM mefenamic acid; 50MEF, 50 µM mefenamic acid; 100MEF, 100 µM mefenamic acid. *p < 0.05 compared to control, **p < 0.05 compared to IR.
Discussion and Conclusion
In this study, we showed priming of human lymphocytes with MEF markedly reduced genotoxicity induced by IR. The frequency of micronuclei was reduced with pretreatment of MEF. MEF is widely used as an anti-inflammatory drug for relieving of pain and inflammation [15]. MEF belongs to NSAID, and the mechanism of action is partially attributed to capability of inhibiting the cyclooxygenase-2 (COX-2) enzyme, an essential component of the inflammatory process [1617]. MEF has an inhibitory effect on the proliferation of cancer cells such as liver and colon cancer cells. Different mechanisms are proposed for anti-cancer effect of MEF to be caspase-3 pathway and inhibiting of the synthesis of hyaluronic acid and cyclooxygenase [18192021]. COX-2 is frequently over expressed in various cancer tissues, thus inhibition of COX-2 by NSAID is suggested as a strategy for cancer treatment and prevention [2021222324]. While inflammatory environment is linked to DNA damage and death in normal cells, proinflammatory cytokines are involved in cancer cell growth [2526]. Inflammatory cytokines secreted by tumor activate the necessary pathways to facilitate cancer cells proliferation [27], It is recommended that blockade of inflammation process by NSAIDs inhibit cancer progression. In the normal cells, exposure to IR initiates secretion of inflammatory cytokines, which is involved in DNA damage [2829]. Mukherjee et al. [30] showed that NSAIDs could reduce radiation-induced chromosomal instability in vivo. In this study we showed that MEF mitigated IR induced DNA damage in human lymphocytes. It is well documented that free radicals and inflammation are main triggers for IRinduced DNA damage. Normal cell is going to dysfunction in inflammatory process. It is cleared that anti-inflammatory effect of MEF by inhibiting of COX-2 and decreasing secretion of cytokines is main proposed mechanisms for radioprotective effect of MEF. Whereas MEF has anti-cancer effect, it likely protects, in vitro , normal cell against IR induced DNA damage. This result provides a new indication of MEF for protection of normal cells during radiation therapy in treatment of cancer patients. Future studies are needed to find the exact mechanism such as detailed pathways for the radioprotective effect of MEF.
Acknowledgments
This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran. This research was the subject of a PharmD thesis of Reyhaneh Nobakht as a PharmD student of Mazandaran University of Medical Sciences.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.