Dose escalated simultaneous integrated boost of gross nodal disease in gynecologic cancers: a multi-institutional retrospective analysis and review of the literature
Article information
Abstract
Purpose
Typical doses of 45–50.4 Gy used to treat regional nodes have demonstrated inadequate control of gross nodal disease (GND) in gynecologic cancer, and accelerated repopulation may limit the efficacy of a sequential boost. We reviewed outcomes of patients treated with a simultaneous integrated boost (SIB) at 2.25 Gy per fraction to positron emission tomography (PET) avid GND to evaluate toxicity and tumor control using this dose-escalated regimen.
Materials and Methods
A total of 83 patients with gynecologic cancer and PET avid inguinal, pelvic, or para-aortic lymphadenopathy were treated using intensity-modulated radiation therapy (IMRT) with SIB. Primary cancers were mostly cervical (51%) and endometrial (34%), and included patients who received concurrent chemotherapy (59%) and/or brachytherapy boost (78%).
Results
Median follow-up from radiation completion was 12.6 months (range, 2.7 to 92.9 months). Median dose to elective lymphatics was 50.4 Gy (range, 45 to 50.4 Gy) at 1.8 Gy/fraction. Median SIB dose and volume were 63 Gy (range, 56.3 to 63 Gy) and 72.8 mL (range, 6.8 to 1,134 mL) at 2–2.25 Gy/fraction. Nodal control was 97.6% in the SIB area while 90.4% in the low dose area (p = 0.013). SIB radiotherapy (RT) field failure-free, non-SIB RT field failure-free, and out of RT field failure-free survival at 4 years were 98%, 86%, and 51%, respectively. Acute and late grade ≥3 genitourinary toxicity rates were 0%. Acute and late grade ≥3 gastrointestinal toxicity rates were 7.2% and 12.0%, respectively.
Conclusion
Dose escalated SIB to PET avid adenopathy results in excellent local control with acceptable toxicity.
Introduction
In gynecologic cancer, involvement of pelvic or para-aortic lymph nodes (LN) is a poor prognostic factor [1]. While radiation to involved nodes has long been shown to increase survival, standard doses (45–50 Gy) often do not control gross nodal disease (GND) [2-4]. Earlier attempts at dose escalation with three-dimensional conformal radiation therapy (3DCRT) posed the problem of excessive toxicity [5].
A sequential boost can be delivered with planned 3DCRT or intensity-modulated radiation therapy (IMRT), which has been shown to mitigate toxicity and allow dose intensification. IMRT can also be used to deliver a simultaneous integrated boost (SIB), increasing dose without increasing overall treatment time (OT) to counter accelerated repopulation [6-8] (Table 1).
Evidence for a GND [4] boost has led to the National Comprehensive Cancer Network (NCCN) guidelines (version 1.2020) for cervical cancer allowing simultaneous or sequential boost [9]. This evolving treatment paradigm is reflected in current trials such as EMBRACE-II, which allows GND to be treated to 55–65 (equivalent dose in 2 Gy fractions [EQD2] including brachytherapy) [10].
Less evidence exists for other gynecologic cancers. In endometrial cancer, the NCCN (version 1.2020) allows a boost for gross residual disease without specifically mentioning nodes or boost type [11]. Vulvar cancer recommendations include an unspecified boost type for GND [12]. Similar guidelines in vaginal and ovarian cancer are not available.
We have experience treating GND with SIB across the gynecologic cancer spectrum and sought to evaluate tumor control and toxicity using an aggressive dose-escalated regimen.
Materials and Methods
A retrospective review was performed on patients with biopsy proven gynecologic cancers with positron emission tomography (PET)-avid para-aortic, pelvic, or inguinal nodes that underwent IMRT with SIB from 2009–2020 at two cancer centers. This study was approved by the Ethical Committee of the Baylor Scott & White Health (No. 017-227). A total of 83 patients were identified for inclusion. SIB dose fractionation schedules were the same for each institution. Patients received concurrent chemoradiation (CRT) or radiotherapy (RT) alone with or without brachytherapy. The clinical target volume (CTV) typically included the bilateral common iliac, external/internal iliac, presacral and obturator nodes based on the primary tumor. If GND involved para-aortic nodes, was near or above the common iliac bifurcation, the para-aortic region was included. Inguinal nodes when gross disease was present or there was vulvar of vaginal involvement. Elective nodal fields received 45–50.4 Gy. The SIB volume included the LN identified on computed tomography (CT) simulation and PET/CT with a 5-mm expansion for the planning target volume (PTV). Total dose to the boost volume was 56.25–63 Gy at 2.25 Gy per fraction for an EQD2 of 57.42–64.31 Gy or a BEDacute of 63.8–69.3 Gy10 assuming an α/β of 10 [7]. Brachytherapy treatments included vaginal cylinder (VC), tandem and ovoid (T&O), and interstitial needles with Syed template (Alpha-Omega Services Inc., Bellflower, CA, USA).
Constraints of bowel bag-CTV (bowel bag excluding the bowel in the CTV) were a maximum point dose of 60 Gy, and a volume of 195 mL or 15% to 45 Gy. Bladder constraints were 15%, 25%, and 50% to 45, 40, and 25 Gy, respectively. Rectum constraints were to 15%, 25%, and 50% to 40, 35, and 25 Gy, respectively. For brachytherapy, the maximal dose to 2 mL of the bladder, rectum, and sigmoid were kept within 70%–75% of the prescribed dose when possible.
All patients received at least one set of post-treatment imaging (PET, CT w/wo contrast, or MRI) 2–3 months after completion of RT (Fig. 1). Post-treatment surveillance PET was only approved by insurance providers for 10 patients. Treatment failures were recorded by location as inside the SIB RT field, inside the non-SIB RT field, inside the RT field (both the SIB and non-SIB fields) or outside of the RT field. Failure-free survival duration was calculated from RT completion to the date of last follow-up or death, at which point patients were censored. Progression-free survival (PFS) and overall survival (OS) were also calculated from RT completion to the date of last follow-up, with death treated as an event.
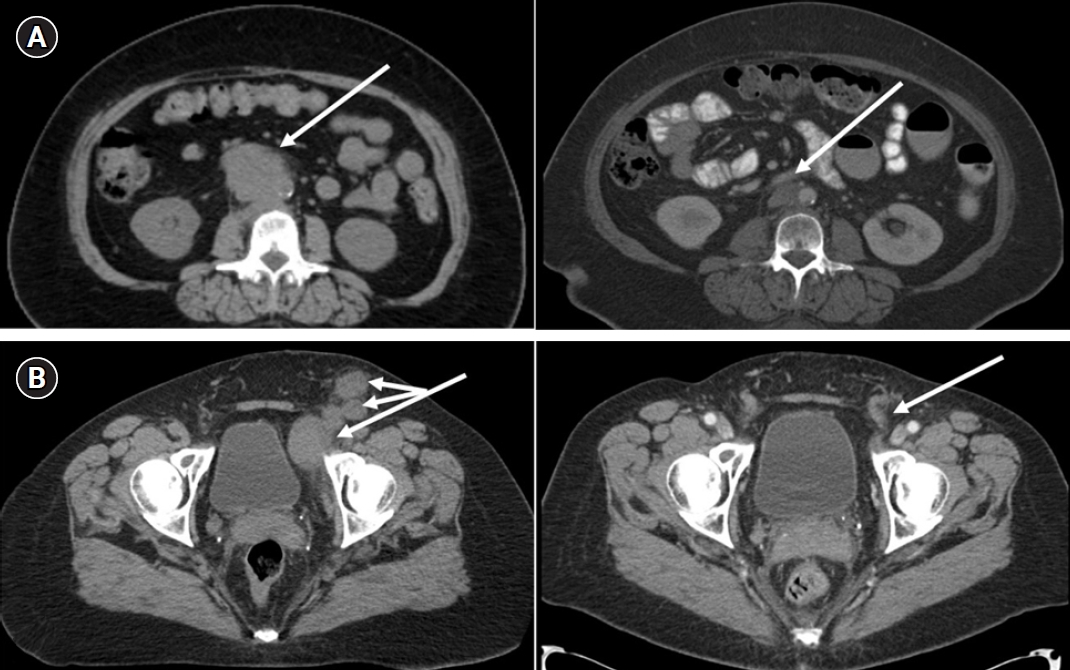
Representative responses. (A) CT simulation (left) and 3-month follow-up (right) of a 66-year-old patient with recurrent endometrial adenocarcinoma at the aortic bifurcation. (B) CT simulation (left) and 4-month follow-up (right) of a 57-year-old patient with poorly differentiated vulvar squamous cell carcinoma following left vulvectomy with multiple bilateral inguinal and pelvic lymph nodes.
Nodal control was defined as either lack of growth or being non-hypermetabolic on PET scan (if performed). Failure was measured in the targeted GND, the remaining treated lymphatics and outside the treated volume. A locoregional recurrence was defined as any recurrence within the RT field. A non-SIB recurrence occurred in the low dose CTV volume outside of the SIB volume. Recurrences outside of the RT field were distant recurrences.
Toxicities were reported using the Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Statistical analysis was done using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
1. Patient and treatment characteristics
The median follow-up was 12.6 months (range, 2.7 to 92.9 months). Table 2 describes patient and treatment characteristics.
2. Control and survival
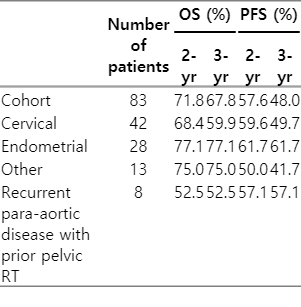
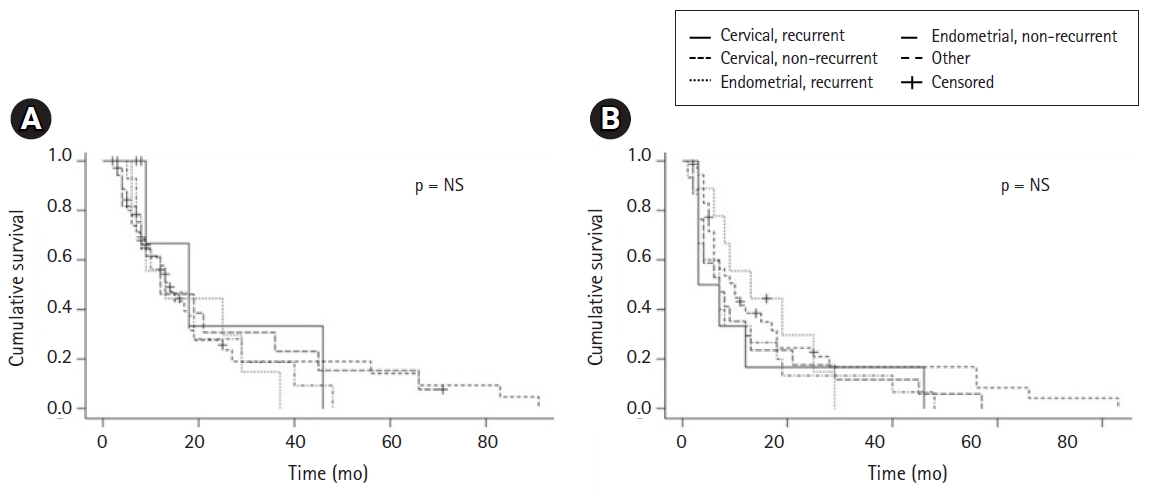
Gross nodal control rate in the SIB RT field was 97.6% (81/83 patients). Only 2 patients (2.4%) progressed in the SIB RT field, while 8 progressed in the non-SIB RT field (9.6%; p = 0.013). SIB failure-free, In field failure-free and out of field failure-free survival at 4 years were 98%, 86%, and 51%, respectively (Fig. 2). PFS and OS rates at 2 years were 58% and 72%, respectively. Median PFS and OS was 19 and 71 months, respectively (Table 3). Cervical cancer patients in the definitive setting had a 2-year PFS and OS of 67% and 72%, respectively. In recurrent patients, 2-year PFS and OS was 20% and 50%, respectively. Endometrial cancer in the primary setting had a 2-year PFS and OS 60% and 72%, respectively. In recurrent patients, 2-year PFS and OS of 67% and 75%, respectively (Fig. 3). Of six vulvar cancer patients, one failed locally in a non-SIB region. Of three vaginal cancer patients, none had local recurrence. All three ovarian cancer patients were treated without debulking surgery after failure on three lines of chemotherapy. One recurred within the SIB field. The only other patient that failed in a SIB region had primary fallopian adenocarcinoma.
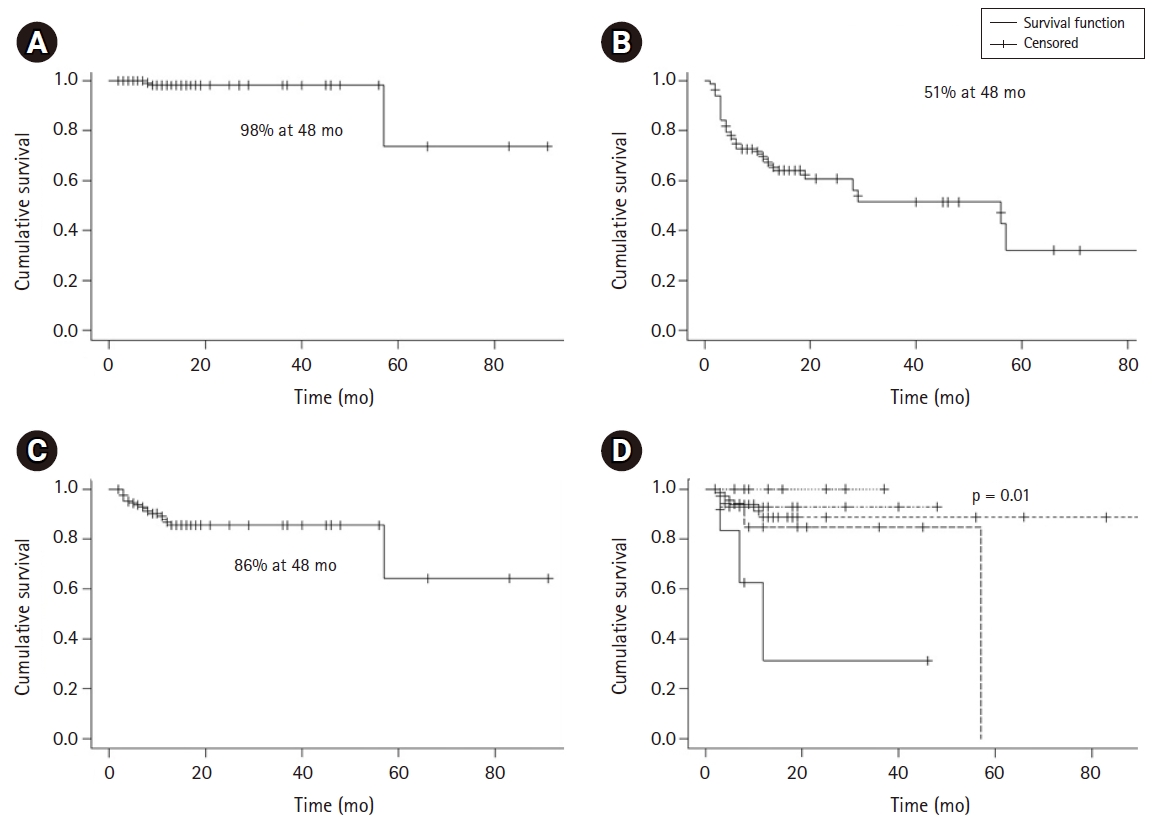
Disease control. (A) Simultaneous integrated boost (SIB) failure-free survival in all patients. (B) Out of field failure-free survival in all patients. (C) In field (non-SIB) failure-free survival in all patients. (D) In field (non-SIB) failure-free survival by type.
There was no difference in survival or recurrence between cervical versus non-cervical cancers or recurrent versus primary cancers (p = 0.9866 and p = 0.6204, respectively).
3. Toxicity
The specifics of non-GI (dermatologic, gynecologic, genitourinary) and GI (gastrointestinal) toxicities were analyzed. Toxicities of G≥3 (grade 3 or greater) were experienced by 10 (12.0%) and 12 (14.5%) patients in the acute and late settings, respectively. The rate of GI toxicities G≥3 in the acute and late settings was 7.2% and 12%, respectively. There were no G≥3 urinary toxicities. The rate of gynecologic toxicities G≥3 in the acute and late settings was 2.4% and 3.6%, respectively. The rate of acute G≥3 dermatologic toxicities was 3.6%. All grade 4 and 5 toxicities were experienced by two patients. The first had a recurrence of a previous pelvic abscess (grade 4 gynecologic toxicity) and developed a small bowel perforation (grade 4 GI toxicity well outside of the SIB field) about 1 month after RT completion. The patient received 50.4 Gy to the elective nodal field with a 63 Gy SIB to para-aortic and pelvic GND along with CRT and T&O boost. The second patient developed enteritis with diarrhea and died of sepsis from ischemic bowl (within the elective field, but well outside of the SIB field) 1 week after completing T&O boost.
4. Boost volume
There was no significant relationship between SIB volume and overall highest toxicity grades (either acute or late; p =0.65). There was no significant difference by SIB volume and occurrence of any acute toxicity or acute toxicity type. A significant relationship was detected by SIB volume and experiencing any acute non-GI (median: 90.7 mL with vs. 37.5 mL without; p = 0.0084) and urinary toxicity (median: 100.8 mL with vs 51.5 mL without; p = 0.02). There was no significant difference by SIB volume for experiencing acute G≥3 toxicity (p = 0.19). Limited incidence prevented analysis by toxicity type. There was no significant difference by SIB volume for experiencing any late toxicity or late toxicity type. There was no significant difference by SIB volume and any (p = 0.58) or GI-only (p = 0.3647) late G≥3 toxicity.
5. Boost dose
There was a significant difference (p = 0.0371) between SIB dose in patients who experienced acute G≥3 toxicities (median 63 Gy and mean 62.3 Gy; 95% confidence interval [CI], 60.8–63.9; range, 48 to 63 Gy) and those who did not (median 63 Gy and mean 59.7 Gy; 95% CI, 58.8–60.6; range, 56.3 to 63 Gy). Split into groups with SIB of <63 Gy (n = 34) versus 63 Gy (n = 49), there was a significant difference in total acute G≥3 toxicity rate (3.0% vs. 18.4%; p = 0.042) but not non-GI (0% vs. 8.2%; p = 0.1405) or GI (2.9% vs. 10.2%; p = 0.39) alone.
There was a significant difference between SIB dose in patients who experienced any or GI-only G≥3 (p = 0.0265 and p = 0.0449, respectively) late toxicities (median, 56.25 Gy; range, 48 to 63Gy) and those who did not (median, 63 Gy; range, 51.75 to 63 Gy). Split into groups of <63 Gy versus 63 Gy, there was a trend toward difference in total late G≥3 toxicity rate (27.6% vs. 8.9%; p = 0.051) but not GI (24.14% vs. 6.82%; p = 0.0776) or non-GI (6.9% vs. 2.3%; p = 0.56).
6. Boost location
SIB to inguinal nodes correlated with higher rates of acute G≥3 dermatologic toxicities (15% vs. 0%; p = 0.0124) and acute G≥3 other toxicities (15% vs. 1.6%; p = 0.042). All other SIB locations (para-aortic, pelvic, inguinal) and toxicity types were not significantly related. Overall highest toxicity grade was not significantly related with SIB to para-aortic (p = 0.68), pelvic (19.6% vs. 33%; p = 0.17) or inguinal nodes (p = 0.55). Vulvar treatment (non-SIB) was significantly associated with acute G≥3 dermatologic toxicity (22.2% vs. 1.35%; p = 0.03) and late G≥3 gynecologic toxicity (25% vs. 1.54%; p = 0.03). There was a significant association between brachytherapy type (none/HDR cuff vs. T&O vs. Syed) and any late G≥3 toxicity (3.5%, 20.0%, and 28%, respectively; p = 0.0328) but not non-GI or GI individually. Two patients treated with SIB to the inguinal nodes only each experienced G≥3 acute and late toxicities. Of the 7 patients who developed a rectovaginal fistula, all had received a brachytherapy boost with 4 (14%) via Syed and 3 (13%) via T&O.
Discussion
We achieved excellent local control (LC) with elective nodal radiation and targeted boost in patients with pathologically enlarged PET positive LNs in both primary and recurrent gynecologic disease. Nodal recurrence was more common in the elective lymphatics than in the grossly positive nodes that received SIB (9.6% vs. 2.4%; p = 0.013), and treatment was well tolerated. With a focus on nodal control, we evaluate our findings by setting and cancer type in the context of the literature (Table 4).
1. Primary cervical cancer
We achieved 100% control of the grossly enlarged LNs in primary cervical cancer patients. Current guidelines and protocols for this population do not recommend a boost method, and the criteria for benefit are not well defined [9,10]. Wakatsuki et al. [13] found that with a sequential boost, recurrence in poor responding patients was correlated with a threshold dose of >58 Gy (56.3% vs. 0%; p = 0.0003). A Singapore study found no significant benefits with a sequential boost [14]. Finally, Choi et al. [15] demonstrated improved PFS (100% vs. 52.4%; p = 0.023), with no difference by LN size or number. While aspects of these studies were compelling, it was not clear whether additional nodal boost with conventional doses offered much benefit. Investigators at Shinshu University concluded that nodal boost may not be necessary in patients with positive nodes restricted to the pelvis, as they found excellent LN control with 3DCRT (87.5%–92%). However, their reported PFS at 2 years of 31.3% is relatively poor compared to other patient cohorts with positive nodes restricted to the pelvis [16]. Using a sequential boost, Yoon et al. [17] reported a 3-year PFS of 59% with IMRT while Ariga et al. [18] reported a 3-year disease-free survival (DFS) of 58% with 3DCRT.
In a more detailed analysis of the dose-volume and intensity effects of nodal boosts in gynecologic cancer, Bacorro et al. [4] demonstrated shorter overall treatment time (by 13 days) in SIB versus sequential boost patients with a trend toward improved nodal control (p = 0.07). They found on univariate analysis that nodal size (volume <3 mL) and dose (EQD2 ≥57.5 Gy) were significant predictors of control. Multivariate analysis confirmed a benefit of treating bulky LNs with increasing dose. Other studies have also identified improved nodal control or OS with increasing dose. The reported LN size thresholds for benefit have ranged from ≥1.5 to 2.4 cm [1,14,19-23]. The number of positive nodes, LN SUV (standardized uptake value) heterogeneity, maximum LN SUV, and various other factors have demonstrated prognostic value [4,18,24-27].
2. Primary endometrial cancer
There is less literature on boosting GND in endometrial cancer. As in cervical cancer, it is unknown what subset of patients would receive the most benefit. Size of LNs has demonstrated an effect on recurrence risk [3,28], with mixed findings on the number of LNs ≥2 [29-32]. Ho et al. [3] showed that patients receiving salvage external beam radiation therapy (EBRT) for nodal recurrences treated above their median dose (64.7 Gy) had improved LC with a trend toward increased DFS (p = 0.099). In their study, 58% of patients received a sequential boost alone or in combination with a SIB. In recurrent or unresectable endometrial cancer, the Harvard group used a median dose of 63 Gy with sequential boost and reported 3-year nodal control and DFS of 86% and 58%. In patients with recurrent disease, 25% (3/12 patients) had nodal relapse, all within the boost field, while in the primary adjuvant setting, 20% (1/5 patients) had a nodal relapse outside the boost field [28]. Boyle et al. [33] used a SIB of 55 Gy at 2.2 Gy per fraction to a heterogenous group of gynecologic malignancies including endometrial cancer, similar to this study, but did not report rates of nodal control. Patients treated for primary endometrial cancer definitively (n = 4) or adjuvantly (n = 15) in our series had 100% gross nodal control. Our results compare favorably to these series and support prospective investigations on dose escalation and sequential boost versus SIB in endometrial cancer.
3. Para-aortic recurrence
Patients with recurrence in the para-aortic lymphatics after primary treatment present a special challenge. Patients with node positive cervical or endometrial cancer treated with RT to the pelvic nodes, with or without extended fields, fail with isolated para-aortic nodal disease at rates of 1.7%–3% [34,35] and 6%–12% [36,37], respectively, though trends toward more frequent follow-up and imaging could increase these rates over time. In cervical cancer for this patient population, 2-year mortality rates can be as high as 100% and locoregional failure up to 50% after para-aortic RT [1,38,39]. As in the primary setting, trends toward improved survival with dose escalation have been reported (3-year OS rates of 58% at ≥51 Gy and 42.8% at ≤50 Gy; p = 0.07) [34]. In endometrial cancer, MD Anderson used SIB with or without an additional sequential boost for isolated para-aortic recurrence and achieved 70% nodal control with a 2-year PFS and OS of 53% and 63%, respectively [40]. We found a 2-year PFS and OS of 52.5% and 57.1% in our small subset of isolated recurrent para-aortic patients. More generally, recurrent cancers throughout our study showed no significant difference in OS, PFS, or nodal control compared to primary cancers. These similar outcomes could be results of equivalent benefit from dose escalation.
4. Other gynecologic malignancies
We had only three patients with ovarian and vaginal cancers, which have limited data on nodal boost outcomes. One Japanese study found that total doses of ≤50 Gy resulted in worse nodal control for vaginal cancer [2]. In our six patients with vulvar cancer, we achieved 100% nodal control without resection, but as with any vulvar radiation, there was significant acute G≥3 dermatologic and late urinary toxicities. A minimum boost dose of 60–70 Gy (type unspecified) has been recommended for GND [12,41,42].
5. Toxicity
Paramount to the discussion of improving LC is how well it is tolerated. In this study, two patients had a grade 4 or 5 toxicity. Each was related to events well outside of the boost fields, making it unlikely that toxicity outcomes would have been different without a boost. Our total acute and late G≥3 toxicity rates of 12% and 16% compare favorably with past cohorts, many of which only reported GI and GU side effects. Overall, prior studies also indicate that using a boost for gross lymphadenopathy is well tolerated.
Historically, patients treated using 3DCRT with or without a sequential boost have largely demonstrated similar toxicity profiles, though one study which used extended-field 3DCRT found increased G≥3 GI toxicity [13-17,43]. IMRT series including nodal boost most frequently report no acute G≥3 GI or GU toxicities, though rates have been reported in small series up to 21.1% and 10%, respectively [24,25,44-48]. The highest rate of GI toxicity occurred in a dose escalation study which treated macroscopic disease (primary tumor and nodes) up to 50 Gy/2.5 Gy fractions and elective nodes up to 45 Gy/2.25 Gy fractions [47]. Late G≥3 toxicities vary widely for both GI (0%–50%) and GU (0%–10%) side effects, with even greater disparity for late grade 1–2 toxicities in cervical and endometrial cancer (GI 0%–78.4% and GU 9.6%–50%) [14,17,25,33,44,45,48,49].
For patients treated for isolated para-aortic recurrence, toxicity from radiation is of increased concern, as many have already received pelvic radiotherapy. In cervical cancer patients treated with a variety of doses and systemic therapies, reported acute G≥3 GI and late G≥3 GU toxicities have ranged from 0% to 7.1% without late G≥3 GI or acute G≥3 GU toxicities [34,38,39]. In endometrial cancer, Shirvani et al. [40] used SIB with or without sequential boost (52% had received prior pelvic RT) and found a G≥3 GI toxicity rate of 18.5%.
Few correlations, and only in the acute setting, were found in our study between SIB volume and toxicity, consistent with other gynecologic studies [25,40]. We also found few significant correlations between G≥3 toxicities and dose ≥63 Gy, which largely correlated anatomically with patients who received primary tumor boosts (Syed, T&O, or vulvar EBRT). Boost dose and technique have not previously demonstrated a significant effect on toxicity, except in one small series [17,21]. A dosimetric analysis has shown significantly reduced dose to the rectum and small bowel using SIB over sequential boost [50]. SIB location also proved to be largely insignificant outside of inguinal nodes, though numbers were too limited to draw any firm conclusions.
Our study is inherently weakened by its retrospective nature and by a short median follow-up length of 12.6 months. The cohort was also heterogeneous with regard to age, cancer type, stage, brachytherapy and systemic therapy. Our analysis of subgroups was limited by small sample size.
In conclusion, we report on the most consistently aggressive radiation regimen for treating grossly positive nodal disease for gynecologic cancer. Using typical dosing of 50.4 Gy at 1.8 Gy per fraction to elective nodes and 63 Gy at 2.25 Gy per fraction to GND, we report high rates of LC compared to prior literature and toxicity rates similar to studies that used lower doses or a sequential boost. Our experience shows favorable outcomes in controlling local disease and contributes to increasing evidence for boosting radiologically positive nodes.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.