 |
 |
AbstractPurposeTo evaluate retrospectively the survival outcome, patterns of failure, and complications in patients treated with postoperative chemoradiotherapy (CRT) in advanced gastric cancer.
Materials and MethodsBetween January 2000 and December 2006, 80 patients with advanced gastric cancer who received postoperative concurrent CRT were included. Pathological staging was IB-II in 9%, IIIA in 38%, IIIB in 33%, and IV in 21%. Radiotherapy consisted of 45 Gy of radiation. Concurrent chemotherapy consisted of a continuous intravenous infusion of 5-fluorouracil and leucovorin on the first 4 days and last 3 days of radiotherapy.
ResultsThe median follow-up period was 48 months (range, 3 to 83 months). The 5-year overall survival, disease-free survival, and locoregional recurrence-free survivals were 62%, 59%, and 80%, respectively. In the multivariate analysis, significant factors for disease-free survival were T stage (hazard ratio [HR], 0.278; p = 0.038), lymph node dissection extent (HR, 0.201; p = 0.002), and maintenance oral chemotherapy (HR, 2.964; p = 0.004). Locoregional recurrence and distant metastasis occurred in 5 (6%) and 18 (23%) patients, respectively. Mixed failure occurred in 10 (16%) patients. Grade 3 leukopenia and thrombocytopenia were observed in 4 (5%) and one (1%) patient, respectively. Grade 3 nausea and vomiting developed in 8 (10%) patients. Intestinal obstruction developed in one (1%).
IntroductionGastric cancer is most common cancer in Korea. The gastric cancer incidence rate was 15.7% of all cancers in 2008 [1]. Surgical resection is the most important treatment modality in gastric cancer patients. However, surgery alone is often insufficient for a cure because of frequent relapse after resection. Many studies have revealed that the significant failure pattern after curative resection of gastric cancer was locoregional recurrence [2-5]. Thus, several adjuvant treatment modalities, including preoperative or postoperative adjuvant chemoradiotherapy (CRT), have been used in attempts to reduce locoregional failure following curative surgery [6-12].
Evidence for the benefit of postoperative adjuvant CRT in patients with locally advanced gastric cancer has been provided in several studies [7,10,11]. The Intergroup 0116 (INT-0116) trial showed that postoperative CRT improved survival over surgery alone in patients with disease extension through the gastric wall and/or regional lymph node (LN) involvement, even though the quality of surgery in that study has been criticized [10]. The 3-year survival rates were 50% in the chemoradiotherapy group and 41% in the surgery-only group. The 3-year rates for relapse-free survival were 48% in the chemoradiotherapy group and 31% in the surgery-only group. Since the report of the INT-0116 trial, postoperative CRT in gastric cancer has become a standard adjuvant treatment in the United States [13]. Additionally, Kim et al. [11] reported that postoperative CRT in 544 gastric cancer patients treated with extensive curative lymph node dissection (D2 dissection) prolonged overall survival and decreased locoregional recurrence.
Our institution has used postoperative adjuvant CRT in patients treated with gastric cancer resection to reduce locoregional failure. The aim of this study was to evaluate retrospectively survival outcomes, patterns of failure, and complications in patients treated with postoperative CRT in advanced gastric cancer.
Materials and Methods1. PatientsBetween January 2000 and December 2006, 80 patients with advanced gastric cancer who received postoperative concurrent CRT were enrolled. Inclusion criteria were patients with stage IB through IV, and who received a radiation dose of more than 30 Gy. The TNM stages were determined according to the 6th edition of American Joint Committee on Cancer (AJCC) cancer staging manual. All patients except six underwent R0 resections. D2 dissection was performed in 74 (93%) patients. Abdominal-pelvic computed tomography (CT) and endoscopy were performed in all cases to evaluate the extent of the disease before the resection. Pretreatment patient characteristics are summarized in Table 1. The gender ratio was 73% males and 27% females. The median age was 56 years (range, 23 to 71 years). The most common histological type was an adenocarcinoma. Pathological staging was IB-II in 9%, IIIA in 38%, IIIB in 33%, and IV in 21%. Informed consent was obtained from all patients before treatment.
2. ChemoradiotherapyAfter undergoing curative or palliative resection, postoperative adjuvant CRT was started approximately at 4 weeks after surgery. Radiotherapy consisted of 45 Gy of radiation at 1.8 Gy/day, 5 days/week, for 5 weeks. The median radiation dose was 45 Gy (range, 32.4 to 59.4 Gy). A boost dose of 5 to 14.4 Gy was delivered after the 45 Gy to patients with positive resection margins or close resection margins. The tumor bed was defined by preoperative abdominal CT imaging and surgical clips. Radiation was delivered with 6 or 15-MV photons. All patients received radiotherapy using an anterior-posterior (AP-PA) opposing field. The radiation field included the tumor bed, anastomosis site, duodenal stump, remnant stomach, and regional nodes with high recurrence risk [14]. However, the radiation field was slightly customized in each patient according to TN stage and performance status. Concurrent chemotherapy consisted of a continuous intravenous infusion of 5-fluorouracil (5-FU, 500 mg/m2) and leucovorin (LV, 20 mg/m2) on the first 4 days and last 3 days of radiotherapy. At 1 month after the completion of CRT, patients were recommended to take daily oral 5-FU derivatives for at least 1 year [15,16].
3. Toxicity and follow-up evaluationTreatment toxicities were evaluated according to the comprehensive criteria for assessing therapy-induced toxicity [17]. Patients with postoperative CRT were followed up at 3-month intervals for the first year, at 6-month intervals for the next 4 years, and annually thereafter. The follow-up evaluation consisted of a physical examination, gastroduodenoscopy, abdominopelvic CT, and positron emission tomography (PET)/CT if necessary. The failure pattern was divided into locoregional, distant, and mixed failure. Locoregional relapse was defined as recurrence at the anastomosis site, remnant stomach, or gastric bed, or a regional lymph node. Distant relapse was defined as peritoneal seeding, liver metastasis, or metastasis of other extra-abdominal sites. Mixed failure was defined as both site recurrence in locoregional and distant site recurrences.
4. Statistical analysisOverall survival (OS) was defined as the time between the operation date and the date of death due to any cause or the date of the last follow-up. Disease-free survival (DFS) was defined as the time between the operation date and the date of any recurrence as the first event. Survival outcomes were calculated using the Kaplan-Meier method. Prognostic factors for OS and DFS were evaluated using the log-rank test as a univariate analysis and the Cox proportional-hazards model as a multivariate analysis. A p-value < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results1. Treatment complianceOf the 80 patients, 78 (97%) patients completed postoperative CRT as planned; two patients stopped treatment due to the nausea and vomiting. One of them stopped radiotherapy at 32.4 Gy, and he underwent a cholecystectomy because of acute acalculous cholecystitis. Despite of the surgery, he finally died a result of an abdominal wall abscess. Another patient experienced grade 3 vomiting and stopped radiotherapy at 34.2 Gy. After postoperative CRT, 30 of the 80 patients started daily oral 5-FU derivatives within 3 months and continued for more than 1 year.
2. Survival and prognostic factorsThe median follow-up period was 48 months (range, 3 to 83 months). The 5-year OS, DFS, and locoregional recurrence-free survival were 62%, 59%, and 80%, respectively (Fig. 1). Median OS and DFS were not reached. In subgroup analysis, the 5-year OS and 5-year DFS were 77% and 50%, respectively in stage IIIA. The 5-year OS and 5-year DFS were 50% and 50%, respectively in patients with IIIB. In the univariate analysis, statistically significant prognostic factors for OS were N stage (p = 0.03), TNM stage (p = 0.043), LN dissection extent (p < 0.001), maintenance oral chemotherapy (p < 0.001), and resection margin status (p = 0.042). Significant prognostic factors for DFS were T stage (p = 0.038), LN dissection extent (p < 0.001), maintenance oral chemotherapy (p = 0.001), and resection margin status (p = 0.029) (Table 2).
In the multivariate analysis, significant factors for OS were N stage (hazard ratio [HR], 0.309; 95% confidence interval [CI], 0.112 to 0.848; p = 0.023), LN dissection extent (HR, 0.172; 95% CI, 0.058 to 0.514; p = 0.002), and maintenance oral chemotherapy (HR, 2.965; 95% CI, 1.389 to 6.33; p = 0.005). Significant factors for DFS were T stage (HR, 0.278; 95% CI, 0.083 to 0.932; p = 0.038), LN dissection extent (HR, 0.201; 95% CI, 0.073 to 0.555; p = 0.002), and maintenance oral chemotherapy (HR, 2.964; 95% CI, 1.414 to 6.215; p = 0.004) (Table 3).
3. Patterns of failureOf the 80 patients, 33 (41%) experienced a recurrence during the follow-up period. Of these patients, 28 (85%) patients showed relapse within 1 year after surgery, and 5 (15%) patients showed a relapse after l year. Locoregional recurrence and distant metastasis occurred in 5 (6%) and 18 (23%) patients, respectively. Mixed failure developed in 10 (13%) patients. The most common recurrence site was the peritoneum. Peritoneal seeding developed in 14 patients (42%) among the 33 relapsed patients. Distant metastasis developed in 24 (48%) patients without maintenance oral chemotherapy whereas it was 4 (13%) patients with maintenance oral chemotherapy (p = 0.002). Resection margin was not associated with locoregional recurrence (p = 0.892) but marginal statistical tendency in distant metastasis (p = 0.091).
4. Treatment toxicitiesGrade 3 or worse adverse effects are summarized in Table 4. The most common toxicity was gastrointestinal. The most common gastrointestinal toxicities were nausea and vomiting. Grade 1-2 nausea and vomiting occurred in 76% of patients. Grade 3 nausea and vomiting developed in eight (10%) patients. Grade 3 hematological toxicities, including leukopenia and thrombocytopenia were observed in four (5%) and one (1%) patient, respectively. One patient experienced intestinal obstruction due to proximal jejunal adhesion and needed a surgical intervention.
Discussion and ConclusionThe rationale for postoperative adjuvant CRT following surgery in advanced stomach cancer is the improvement of locoregional control and survival outcome. Several studies have reported both survival benefits and locoregional control in postoperative CRT groups compared with surgery alone. The INT-0116 trial showed significant improvement in the 3-year survival rate (50% vs. 41%) and relapse-free survival (48% vs. 31%) in the CRT group compared with surgery alone [10]. The larger case-control study conducted by Kim et al. [11] showed similar results. In that study, 544 patients received postoperative 5-FU based CRT after curative D2 resection compared with 446 patients receiving surgery alone. The median OS was significantly longer in the CRT group than in the surgery alone group (95.3 vs. 62.6 months). The 5-year OS and 5-year DFS were 57.1% and 54.5%, respectively. Postoperative adjuvant CRT increased the median duration of relapse-free survival significantly (75.6 vs. 52.7 months). The 5-year OS rates in stages IIIA and IIIB were 61.6% and 40.8%, respectively. Our study showed comparable results to that of the INT-0116 study [10] and the Kim et al. [11] studies. In our study, 3-year OS and 3-year DFS were 63% and 62%, respectively. The 5-year OS and 5-year DFS were 62% and 59%, respectively. In our study, more patients with stage III were enrolled compared with the Kim et al. [11] study (71% vs. 46%). Although our study included more patients at a more advanced stage, survival outcome showed comparable results compared to that of the previous studies. Although our results cannot directly compare with previous case-control studies because we didn't include control group, the long-term survival outcomes suggest that postoperative adjuvant CRT may improve OS and DFS in advanced gastric cancer.
One point of difference between our study and the others was maintenance 5-FU based oral chemotherapy. The patients were recommended to take oral 5-FU based drugs for 1 year after the adjuvant CRT. A large-scale trial has compared postoperative adjuvant therapy with or without daily oral 5-FU derivatives. In that study, oral chemotherapy was started within 6 weeks after surgery and continued for 1 year. The results showed that adjuvant oral chemotherapy was an effective adjuvant treatment for patients who had undergone a D2 dissection for locally advanced gastric cancer without severe toxicity [15,16]. In our study, 30 patients completed maintenance oral chemotherapy over 1 year. Maintenance oral chemotherapy was a significant prognostic factor for OS and DFS in the univariate and multivariate analysis. These finding suggest that maintenance oral chemotherapy using oral 5-FU can improve survival after postoperative CRT in resected gastric cancer. The reason of survival benefit using oral maintenance chemotherapy inferred from a decrease of the distant metastasis. However number of patients who completed maintenance oral chemotherapy over 1 year were small, large scale study will be necessary to determine the definitive role of maintenance oral chemotherapy.
Peritoneal seeding is one of the most common causes of failure after curative surgery for gastric cancer [18-22]. Peritoneal recurrence is generally not surgically treatable, and the efficacy of salvage chemotherapy is limited. Thus, the average survival duration after peritoneal seeding is only approximately 6 months [19]. Kim et al. [11] reported that peritoneal recurrence was the most frequent pattern of failure following adjuvant chemoradiotherapy after curative resection with extensive (D2) lymph node dissection. The locoregional failure rate was 14.9%. This result was similar to our result of 19% locoregional failure. In an attempt to prevent peritoneal recurrence, intraperitoneal hyperthermic chemoperfusion (IHCP) treatment has been combined with aggressive surgery. Treatment using the intraperitoneal chemotherapy under normothermic or hyperthermic conditions has shown encouraging results [23-25]. But further studies are necessary to prevent peritoneal recurrence following curative resection of advanced gastric cancer.
In the INT-0116 [10] and Kim et al. [11] studies, grade 3-4 hematological toxicity occurred in 54% and 30% of patients, respectively, and grade 3-4 gastrointestinal toxicity in 33% and 15%, respectively. In our study, grade 3-4 hematological and gastrointestinal toxicities occurred in 6% and 10% of patients, respectively. One possible reason that these treatment toxicities were much lower in our study may be the duration of chemotherapy. The INT-0116 [10] and Kim et al. [11] studies used a total of five cycles of 5-FU/LV chemotherapy. In those studies, the combined-modality group received radiotherapy of 45 Gy in 25 fractions, concurrent with two cycles of 5-FU/LV chemotherapy. Additional three cycles of 5-FU/LV chemotherapy were given to patients before and after CRT. Compared with those studies, we used only two cycles of 5-FU/LV chemotherapy during radiotherapy. We suspect that the difference in the total number of chemotherapy cycles resulted in the decreased treatment toxicities.
The lymphatic system in the stomach is complex. There is substantial controversy about the appropriate extent of the lymph dissection. D2 dissection has been accepted as the standard surgical approach in Korea and Japan, whereas D1 dissection is favored in Western countries. Two randomized trials, the Dutch Gastric Cancer Trial (DGCT) and the trial of the UK Medical Research Council (MRC), showed that D2 dissection did not improve survival compared with D1 dissection [26,27]. Additionally, the INT-0116 study showed no significant difference in relapse-free or OS according to the extent of LN dissection (p = 0.80) [10]. Contrary to previous results, the 15-year follow-up data from the DGCT showed that D2 lymph adenectomy was associated with lower locoregional recurrence and gastric-cancer-related death rate than D1 surgery in Western patients with gastric cancer, because a safer, spleen-preserving D2 resection is currently available [28]. Recent study of Dikken et al. [29] demonstrated that addition of postoperative CRT after D1 surgery decreased local recurrence in resectable gastric cancer (2% vs. 8%; p = 0.001). In our study, LN dissection extent was a significant OS and DFS prognostic factor in both univariate and multivariate analysis. However the number of patients of D1 dissection group was too few. Therefore further study including a larger number of patients is necessary to clarify the prognostic significance of LN dissection extent.
Surgical resection is the most important treatment for patients with advanced gastric cancer. Achieving microscopically negative margin (R0) around the tumor is the principal goal of surgery because even minimal remaining cancer cells cause locoregional recurrence. Wang et al. [30] study revealed that positive resection margin is one of the independent unfavorable factors for OS in gastric cancer patients after gastrectomy. Regarding recurrence pattern for patients with positive resection margin, the most common recurrence was distant metastasis. Similar to Wang et al. [30] study, positive resection margin was significant an unfavorable factor for OS (p = 0.042) and DFS (p = 0.029) in our study. Resection margin status was not a significant factor for locoregional recurrence rather than may be associated with distant metastasis. This result may be due to the effect of postoperative adjuvant CRT including boost dose in case of positive resection margin. Therefore aggressive postoperative CRT following surgery would decrease the locoregional recurrence in patients with positive resection margin.
The limitation of this study is that it was a retrospective single arm study without surgery alone group. In addition, small number of patients makes difficult to interpret in subgroup analysis according to LN dissection extent and resection margin.
In summary, survival results of our retrospective study were comparable to those reported in other postoperative radiation therapy trials so far. Our postoperative CRT regimen seems to be a safe and effective method, reducing locoregional failure without severe treatment toxicity in advanced gastric cancer patients.
References1. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011;43:1–11, PMID: 21509157.
2. Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1–11, PMID: 7061243.
3. Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys 1990;19:1357–1362, PMID: 2262358.
4. McNeer G, Vandenberg H Jr, Donn FY, Bowden L. A critical evaluation of subtotal gastrectomy for the cure of cancer of the stomach. Ann Surg 1951;134:2–7, PMID: 14838535.
5. Thomson FB, Robins RE. Local recurrence following subtotal resection for gastric carcinoma. Surg Gynecol Obstet 1952;95:341–344, PMID: 14950669.
6. Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol 2002;80:72–78, PMID: 12173383.
7. Leong CN, Chung HT, Lee KM, et al. Outcomes of adjuvant chemoradiotherapy after a radical gastrectomy and a D2 node dissection for gastric adenocarcinoma. Cancer J 2008;14:269–275, PMID: 18677137.
8. Reed VK, Krishnan S, Mansfield PF, et al. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2008;71:741–747, PMID: 18164837.
9. Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC): report on 370 patients. Int J Radiat Oncol Biol Phys 1998;42:929–934, PMID: 9869212.
10. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725–730, PMID: 11547741.
11. Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005;63:1279–1285, PMID: 16099596.
12. Lim DH, Kim DY, Kang MK, et al. Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist's view. Br J Cancer 2004;91:11–17, PMID: 15162146.
13. Macdonald JS. Adjuvant therapy for gastric cancer. Semin Oncol 2003;30(4 Suppl 11):19–25, PMID: 14506600.
14. Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol 2002;12:187–195, PMID: 11979420.
15. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–1820, PMID: 17978289.
16. Nakajima T, Takahashi T, Takagi K, Kuno K, Kajitani T. Comparison of 5-fluorouracil with ftorafur in adjuvant chemotherapies with combined inductive and maintenance therapies for gastric cancer. J Clin Oncol 1984;2:1366–1371, PMID: 6439835.
17. Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH. Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 1990;8:147–159, PMID: 2400936.
18. Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol 1992;1:341–346, PMID: 1341269.
19. Averbach AM, Jacquet P. Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer. Br J Surg 1996;83:726–733, PMID: 8696727.
20. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236–242, PMID: 10671934.
21. Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg 2000;87:353–357, PMID: 10718807.
22. Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003;90:1113–1119, PMID: 12945079.
23. Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 2001;25:985–990, PMID: 11571980.
24. Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529–534, PMID: 10091726.
25. Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 2001;48:1776–1782, PMID: 11813623.
26. Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–914, PMID: 10089184.
27. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522–1530, PMID: 10188901.
28. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–449, PMID: 20409751.
29. Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430–2436, PMID: 20368551.
30. Wang SY, Yeh CN, Lee HL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol 2009;16:2738–2743, PMID: 19636636.
Fig. 1Kaplan-Meyer curves of overall survival (OS), disease-free survival (DFS), and locoregional recurrence-free survival (LRFS). The 5-year OS was 62%. Median OS was not reached. The 5-year DFS was 59%. Median DFS was not reached. The 5-year LRFS was 80%. 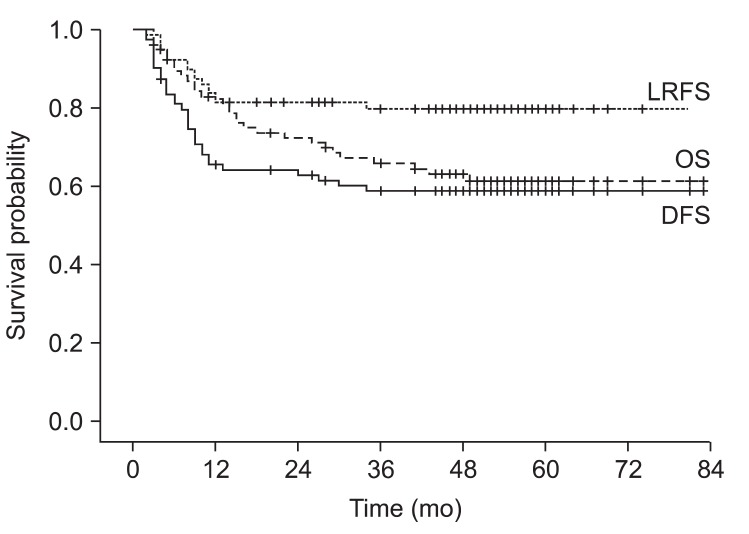
|
|
||||||||||||||||||||||||||||||||||||

 |
 |