 |
 |
AbstractPurposeThe study aims to report late toxicities in locally advanced head-and-neck squamous cell carcinoma (LAHNSCC) treated with intensity-modulated radiation therapy (IMRT).
Materials and MethodsA retrospective study was conducted on 103 patients of LAHNSCC treated with IMRT. We analyzed the cumulative incidence of late xerostomia, dysphagia, and aspiration at an interval of 6-month, 1-year, 2-year, and 3-year from the start of IMRT.
ResultsAt a median follow up of 4.2 years (interquartile range, 3.5 to 6 years), the cumulative incidence of grade ≥2 late xerostomia was 5.5%, dysphagia was 6.9%, and aspiration was 11.1%. Logistic regression showed that Dmean of ≥26 Gy to parotids had higher risk of xerostomia (hazard ratio [HR] = 5.19; 95% confidence interval [CI], 1.90–14.22; p = 0.001). Late dysphagia was associated with Dmean of ≥45 Gy to pharyngeal constrictors (PC) (HR = 7; 95% CI, 1.84–26.61; p =0.004), ≥55 Gy to larynx (HR = 3.25; 95% CI, 1.15–9.11; p = 0.025), and adjuvant RT (HR = 5.26; 95% CI, 1.85–14.87; p = 0.002). Aspiration was associated with Dmean of ≥45 Gy to larynx (HR = 6.5; 95% CI, 1.93–21.88; p = 0.003), Dmean of ≥55 Gy to PC (HR = 3.54; 95% CI, 1.25–9.98; p = 0.017), and patients having late dysphagia (HR = 4.37; 95% CI, 1.55–12.31; p = 0.005).
IntroductionSurgery and radiation therapy (RT), along with chemotherapy, is the current standard of care in locally advanced head-and-neck squamous cell carcinoma (LAHNSCC) [1,2]. The delivery of RT in head-and-neck cancer (HNC) has always been a challenge due to the proximity of critical organs and target volumes, resulting in intractable treatment-related toxicities. The RT technique has changed over time from two-dimensional RT to more conformal techniques. Intensity-modulated radiation therapy (IMRT) has gained popularity in contemporary clinical practice due to superior dose conformity [3]. IMRT is associated with decreased incidence and severity of xerostomia compared to three-dimensional conformal radiation therapy (3DCRT) [4-6]. Also, patients receiving definitive IMRT have a significantly lesser duration of dysphagia and feeding tube placement than 3DCRT [7]. The quality of life improved with IMRT compared to 3DCRT [8]. The randomized controlled trials (RCTs) suggest IMRT has similar locoregional control (LRC) and overall survival (OS) compared with 3DCRT [4-6]. A Surveillance, Epidemiology and End Results (SEER)-Medicare analysis showed that IMRT is associated with improved cause-specific survival (CSS) compared to non-IMRT in HNC [9]. The patterns of care study suggest IMRT use in HNC to be safe in community practice [10].
Despite all the advantages, the practice of IMRT is disputable. A learning curve exists for IMRT practice in HNC, and the experience of treating oncologists is related to cancer outcomes. The RTOG-0022 study reports a higher failure in oropharyngeal cancer patients with major IMRT protocol violations [11]. A high-volume provider has decreased all-cause mortality, aspiration pneumonia, and better OS than low-volume providers of IMRT in HNC. The key finding of this population-based study is the impact of the experience of IMRT providers. The mortality risk decreased by 21% for additional five patients per year [12]. The non-availability of IMRT facilities, increased travel time for RT, and poor referral patterns may hinder patients from taking treatment at a high-volume regional center. Hence, the current focus is training in contouring and strict adherence to implementing IMRT in HNC. The various cooperative group has published guidelines and atlas for better delineation of target and organ-at-risk (OAR) [13,14]. Xerostomia, dysphagia, and aspiration being the most troublesome side effects, it’s imperative to restrict doses to salivary glands and dysphagia aspiration-related structure (DARS), whenever feasible [15,16]. Most literature on dose constraint is based on retrospective series and expert opinion [17].
The RCTs primarily report xerostomia, local control, and overall survival [4-6]. The other late toxicities such as dysphagia and aspiration are reported from single institute series and are limited by a small sample size [18-27]. We conducted an audit to review our experience given limited data availability on late toxicities except for xerostomia and the intricacies associated with IMRT. We have previously published our experience in acute toxicities, mucositis pain, acute mortality, and concurrent chemoradiation (CRT) in patients with HNC [28-31]. The present study aims to report the late toxicities in LAHNSCC treated with IMRT.
Materials and Methods1. Study design and settingAfter institute ethical clearance, all HNC patients treated in the Department of Radiation Oncology at St. John's Medical College and Hospital (No. 230/2017) between January 2013 to June 2017 were retrospectively analyzed. The disease had to be squamous cell carcinoma (SCC), treated curatively with IMRT technique (radical or adjuvant), and locally advanced (stage group III, IVA, and IVB). According to the American Joint Committee on Cancer (AJCC) 8th edition, the cancer staging was reconstructed [32]. The exclusion criterion includes patients receiving palliative RT, 3DCRT technique, early-stage HNC (stage I and II), and non-squamous cell histology, including nasopharyngeal carcinoma. All patients were evaluated in a multidisciplinary tumor board. A thorough clinical and endoscopic evaluation was done for all patients. A baseline imaging was done with either computed tomography (CT), positron emission tomography (PET), or magnetic resonance imaging. A biopsy or fine-needle aspiration cytology was performed before starting treatment. A pre-treatment baseline complete blood count, renal function test, liver function test, and creatinine clearance were done.
2. Intensity-modulated radiation therapyA custom-made thermoplastic four-clamp mask with an appropriate headrest was used for immobilization. All patients underwent a contrast-enhanced CT simulation with 2.5 mm slice thickness from vertex to the carina. The segmentation was done on the MONACO workstation. In the definite setting, gross tumor volume (GTV), high-risk clinical target volume (CTV1), intermediate-risk CTV (CTV2), and low-risk CTV (CTV3) were defined [33]. High-risk planning target volume (PTV1), intermediate-risk PTV (PTV2), and low-risk PTV (PTV3) were generated with an isotropic expansion of 3–5 mm from CTV1, CTV2, and CTV3, respectively. CTV2 was omitted as per treating radiation oncologist’s discretion. The target volumes, i.e., PTV1, PTV2, and PTV3, were irradiated to a total dose of 66–70 Gy, 60–63 Gy, and 54–56 Gy in conventional fractionation, respectively. In the adjuvant setting, only two volumes were defined: CTV1 included tumor bed and CTV2 elective nodal areas. An isotropic expansion of 3–5 mm from CTV is given to generate respective PTV. PTV1 and PTV2 received a dose of 60–66 Gy and 50–54 Gy, respectively. All OAR structures were contoured, such as parotids, submandibular glands (SMGs), pharyngeal constrictors (PC), larynx, and cervical esophagus (CE). The PC was contoured from the pterygoid plates to the inferior border of the cricoid cartilage. The CE was contoured from the lower end of the PC to the lower edge of the C7 vertebral body. The dose constraints used were: spinal cord Dmax <44 Gy; brainstem Dmax <54 Gy; parotid Dmean <26 Gy; SMG Dmean <35 Gy; PC Dmean <45 Gy; larynx Dmean <45 Gy; and CE Dmean <45 Gy. A seven-field IMRT plan with 6 MV photons was generated using the MONACO treatment planning system (Elekta Instrument, Stockholm, Sweden). The typical fields placed were 0°, 45°, 105°, 155°, 205°, 255°, and 315°. The best plan was generated by inverse planning and progressive iterative optimization to ensure optimum coverage of the PTV with an acceptable dose to OARs. Dynamic IMRT was delivered on an Elekta Synergy linear accelerator. Pre-treatment verification was performed by measuring dose maps using the iMatrix. The measured dose distribution data was compared with TPS calculated data by evaluating the gamma index criterion of 3% and 3 mm, i.e., dose difference of <3% and distance to agreement of <3 mm.
3. ChemotherapyIn a radical setting, all patients deemed fit received CRT. Only patients with an extra-capsular extension or a positive margin received CRT in the adjuvant setting [1,2]. The CRT schedule was either weekly or 3-weekly Cisplatin, as decided by the treating medical oncologist. Cisplatin was given at a dose of 40 mg/m2 in a weekly schedule and 100 mg/m2 in a 3-weekly schedule. The maximum numbers of chemotherapy cycles were 7 and 3 in the weekly and 3-weekly schedule, respectively. The number of chemotherapy cycles was titrated based on patient tolerance. Hydration, anti-emetics, and dose modifications were done according to department protocol. Chemotherapy was not given after the completion of RT.
4. Follow-upAll patients were reviewed at least twice a week during therapy for treatment-related toxicities. Patients were admitted for supportive care when indicated. After completion of scheduled treatment, patients were followed up weekly till acute reactions subsided, then monthly till 3 months, every 3 months till 2 years, and yearly after that. A repeat imaging, either CT or PET/CT scan, was performed after 8–12 weeks of RT completion for response assessment. Salvage surgery and/or re-irradiation were planned when feasible. The data collection was done by reviewing radiotherapy charts and outpatient department records.
5. Study outcomeThe primary objective of the study was to report the late toxicities like xerostomia, dysphagia, and aspiration at 6 months, 1 year, 2 years, and 3 years of starting RT. Toxicity scoring was done with Common Terminology Criteria for Adverse Events version 4.03 (CTCAE 4.03) [34]. The late toxicities were not recorded when a patient had a locoregional disease. The mean dose (Dmean) to salivary glands (parotids and SMGs) and DARS (PC, larynx, CE) were recorded.
The secondary objectives were to report treatment compliance, response rates, LRC, OS, and predictors for OS. LRC was defined as no progression or recurrence at the local or regional nodal site. Time to locoregional failure (LRF) was calculated from the date of diagnosis to the date of locoregional disease progression or recurrence. The OS is defined from the time of diagnosis to death due to any cause.
6. Statistical analysisThe data were analyzed using SPSS version 24 (IBM, Armonk, NY, USA). All categorical data were summarized using frequency and percentages. All continuous data were described using the median and interquartile range (IQR) or mean and standard deviation based on the distribution. The late toxicities were presented using cumulative incidence. Logistic regression was used to correlate the Dmean of salivary glands and the risk of late xerostomia. The risk of developing late dysphagia was correlated with the dose received by DARS, presence or absence of acute aspiration, and in adjuvant versus radical group, using logistic regression analysis.
Similarly, the risk of developing late aspiration was correlated with the dose received by DARS, adjuvant versus radical RT, and presence or absence of acute aspiration and late dysphagia. The risk is represented with a hazard ratio (HR) with a 95% confidence interval (CI). Kaplan-Meier method was used to plot LRC and OS. The time to local recurrence and overall survival is represented in median months with a 95% CI. The Cox proportional hazard analysis was used to determine predictors of OS. A cut-off p-value of <0.05 was considered statistically significant.
Results1. Baseline characteristicsA total of 166 HNC patients were treated from January 2013 to June 2017. 103 patients who fit into our inclusion criteria were analyzed (Table 1). The median age and Charlson Comorbidity Scores were 59 years (IQR, 48.5 to 66.5) and 4 (IQR, 3 to 5), respectively. The median weight before starting RT was 59 kg (IQR, 49.5 to 66.5). An advanced tumor (T3 and T4) and a nodal (N2 and N3) stage constituted 71.8% (74/103) and 46.6% (48/103) patients, respectively. The median GTV in the radical group was 58.2 mL (IQR 20.73 to 65.81) as contoured on the RT planning scan. A total of 74.7% of patients (n = 77) were tobacco users, either tobacco chewing or smoking.
At baseline, 14 patients (carcinoma larynx 11 and carcinoma hypopharynx 3) had a tracheostomy tube in situ, and nine patients had clinical features of aspiration. A total of 44 patients had dysphagia, and six patients required a feeding tube (nasogastric tube 5 and feeding jejunostomy 1) before starting RT. None of the patients had xerostomia. Forty-two patients required analgesia (7 required opioids) at baseline. Sixty-eight patients received radical RT, and the rest 35 adjuvant RT. Seventy patients (56 in the radical and 14 in the adjuvant RT group) received CRT. Only cisplatin was used in CRT. A total of 35 and 24 patients received weekly and 3-weekly cisplatin schedules, respectively.
2. Compliance and acute toxicitiesThe median radiation dose received was 66 Gy (IQR, 60 to 66). The median patient volume receiving 60 Gy was 380.38 mL (IQR, 161.49 to 559.8). The median duration of RT was 6.3 weeks (IQR, 5.6 to 6.7). Twenty-four patients (23.3%) did not receive the planned RT dose (17 due to toxicities, 2 had disease progression during RT, and 5 patients discontinued the treatment). Fifteen patients (14.6%) had a gap in the radiotherapy treatment for ≥2 days. The reasons were acute toxicities in 13 patients and machine breakdown in 2 patients. Overall, 64.3% (45/70) patients received ≥200 mg/m2 of cisplatin.
The grade 3 mucositis, dysphagia, pain, aspiration, and dermatitis occurred in 31.1% (32/103), 51.4% (53/103), 15 (14.6%), 13.6% (14/103), and 3.85% (4/103), respectively. Seven patients developed grade 4 aspirations. The median weight loss was 4 kg (IQR, 1 to 6). Fifty-nine patients (57.3%) required either a nasogastric feeding tube (n = 31) or intravenous hydration at daycare (n = 54). Thirty-two patients (31.1%) required admission for supportive care, with a median duration of 12 days (IQR, 5 to 15). The acute xerostomia was not recorded.
3. Late toxicities and dose to OARsThe late toxicities were evaluated in 72 eligible patients with a minimum follow-up of 6 months. The cumulative incidence of xerostomia, dysphagia, and aspiration at 6 months, 1 year, 2 years, and 3 years after starting treatment are enumerated in Table 2. The Dmean (median and IQR) received by OARs were: parotids 25.62 Gy (IQR, 24.25 to 33.33), SMGs 53.72 Gy (IQR, 29.45 to 59.50), PC 48.56 Gy (IQR, 42.92 to 55.26), CE 42.59 Gy (IQR, 31.68 to 46.74), and larynx 47.77 Gy (IQR, 40.42 to 56).
Most patients developed xerostomia, but it subsided in more than half of patients by the end of 6 months (Table 2). None of the patients developed grade 3 xerostomia, and the majority had grade 1 toxicity. The grade 2 xerostomia occurred in four patients (5.5%) at 6 months, three patients (5.4%) at 1 year, one patient (2.4%) at 2 years, and none at 3 years. The Dmean of ≥26 Gy to parotids was associated with a significantly higher risk of xerostomia (HR = 5.19; 95% CI, 1.90–14.22; p = 0.001). Similarly, there was an increasing trend of developing xerostomia, when Dmean of SMGs was ≥35 Gy (HR = 2.36; 95% CI, 0.85–6.56; p = 0.099).
More than half of the patients (51.4%) either required tube feeding or intravenous hydration during treatment period. Only three patients had grade 3 dysphagia and were feeding tube dependent at 6 months. Rest 24 patients with dysphagia managed oral intake with dietary modifications. The grade ≥2 dysphagia occurred in five patients (6.9%) at 6 months, three patients (5.4%) at 1 year, two patients (4.7%) at 2 years, and one patient (3%) at 3 years (Table 2). With logistic regression analysis, the risk of late dysphagia had a significant association with Dmean of ≥45 Gy to PC (HR = 7; 95% CI, 1.84–26.61; p =0.004), Dmean of ≥55 Gy to larynx (HR = 3.25; 95% CI, 1.15–9.11; p = 0.025), and patients receiving adjuvant RT (HR = 5.26; 95% CI, 1.85–14.87; p = 0.002). There was a trend of increased risk of dysphagia with Dmean of ≥45 Gy to CE (HR = 2.55; 95% CI, 0.93– 6.96; p = 0.067) and ≥45 Gy to larynx (HR = 2.27; 95% CI, 0.82–6.24; p = 0.112). The severity of acute dysphagia had no correlation in developing late dysphagia (HR = 1.66; 95% CI, 0.63–4.36; p = 0.302) (Supplementary Table S1).
One-thirds of patients had aspiration at end of 6 months, which decreased to half at 1 year (Table 2). The majority (n = 17) had grade 1 aspiration. The grade ≥2 aspiration occurred in eight patients (11.1 %) at 6 months, two patients (3.6%) at 1 year and none at 2 and 3 years. Patients with grade ≥2 aspiration received multiple antibiotic courses or had a hospital admission for a respiratory infection. The late aspiration pneumonia was recorded as cause of death in four patients. The risk of aspiration is associated with Dmean of ≥45 Gy to larynx (HR = 6.5; 95% CI, 1.93–21.88; p = 0.003), ≥55 Gy to PC (HR = 3.54; 95% CI, 1.25–9.98; p = 0.017). Also, the patients with late dysphagia were at higher risk of developing aspiration (HR = 4.37; 95% CI, 1.55–12.31; p = 0.005). Patients receiving adjuvant RT had higher trend of developing late aspiration compared to radical RT (HR = 2.65; 95% CI, 0.97–7.17; p = 0.056). The presence of acute aspiration was not associated with late aspiration (HR = 0.63; 95% CI, 0.23–1.66; p = 0.348) (Supplementary Table S1).
Among other late toxicities, pain, subcutaneous fibrosis, and neck edema were observed in 13 patients (18.1%), 27 patients (37.5%), and 28 patients (38.9%), respectively. The majority were grade 1 toxicities. Only one patient was reported with osteoradionecrosis (ORN). The patient was a case of recurrent carcinoma buccal mucosa, which required surgical release for trismus and debridement for ORN.
4. Disease control and survivalThe median follow-up was 4.2 years (IQR, 3.5 to 6) in surviving patients. Response assessment was done after 10–12 weeks of RT completion. In the definite group, 57.3% of patients (39/68) achieved a CR. Salvage surgery was performed for only one patient, and two patients received palliative chemotherapy. In the adjuvant group, all except one patient (34/35) was free of disease. At the time of analysis, 30 patients had recurred. The median time to LRF was 33 months (95% CI, 1–76) (Fig. 1A). The recurrence pattern was local, regional nodal, local with regional nodal, locoregional with distal metastasis, and distal metastasis alone in 14, four, five, three, and four patients, respectively. Most patients had an unresectable disease at recurrence. Only six patients were suitable for salvage surgery. At recurrence, one patient underwent surgery alone, three patients underwent surgery and adjuvant re-irradiation, one patient received re-irradiation with concurrent chemotherapy, five patients received palliative chemotherapy, four patients received alternate therapy, and the majority (n = 14) received best supportive care. Two patients refused salvage surgery.
A total of 74 patients had died at the time of analysis. A total of 75.6% of deaths (56/74) occurred in the first 2 years, and most deaths were due to LAHNSCC (n = 38). Acute toxicities and sepsis caused deaths in 12 patients in the first year. Nine deaths were due to intercurrent illness (Supplementary Table S2). Three patients developed second primary (one each of carcinoma buccal mucosa, carcinoma anterior tongue, and carcinoma cervix). Among 29 alive patients at the last visit, 24 patients had more than 4.5 years of follow-up. The median OS was 19.26 months (95% CI, 14.7–23.8) (Fig. 1B). The Cox proportional hazard model analysis showed the OS improved with surgery and adjuvant RT compared with radical RT ± chemotherapy (HR = 1.69; 95% CI, 1.02–2.79; p = 0.040). The OS worsened with stage IVA and IVB compared with stage III (HR = 1.83; 95% CI, 1.11–3.03; p = 0.017) (Supplementary Table S3).
Discussion and ConclusionThe cumulative incidence of grade ≥2 xerostomia, dysphagia, and aspiration at 1 year were 5.4%, 5.4%, and 3.6%, respectively. A Dmean of ≥26 Gy to the parotids was associated with a higher risk of xerostomia. Dysphagia was found to be significantly associated with Dmean of ≥45 Gy to the PC, ≥55 Gy to the larynx, and a patient receiving adjuvant RT. The risk of aspiration is associated with Dmean of ≥45 Gy to larynx and patients having late dysphagia. After a median follow-up of 4.2 years, the median time to LRF and OS was 33 months and 19.26 months, respectively.
The incidence of grade 1, 2, and 3 xerostomia varied from 13.1%–42%, 10%–19.7%, and 0%–1.6%, respectively at 1 year after IMRT [21,23,26,27]. The incidence of xerostomia decreased until 1 year but remained stable 2 years after IMRT [27,35]. The grade ≥1 xerostomia was associated with a mean dose to the contralateral parotid glands ≥26 Gy [23]. The literature recommends restricting the mean dose to 26 Gy for at least one parotid gland and attempts to reduce the doses to contralateral SMG [17]. We report similar incidence and severity of xerostomia and a decreasing trend of xerostomia years after IMRT. The mean dose of <26 Gy to parotid glands was achieved, but a decreasing dose to SMGs was not feasible in our study population.
The reporting of dysphagia is also varied. Grade 3 dysphagia (feeding tube dependent) was reported in 13% of patients at 1 year by Montejo et al. [20]. Mazzola et al. [23] reported grade 2 dysphagia at 6, 12, and 24 months in 26% (n = 9), 23% (n = 8), and 23% (n = 8), respectively. Baudelet et al. [35], reported 60% of patients experienced dysphagia (grade 1–4) at 1 year, and decreasing to 36% at 5 years. The majority were grade 1, and less than 10% were grade 2 or more. We report a lesser incidence of dysphagia, 27.3% and 23.8% at 1 year and 2 years, respectively. In a review by De Felice et al. [36], the late dysphagia was associated with Dmean of >56 Gy to the larynx, >63 Gy to PC, and >48 Gy to CE. In our study, the Dmean received by the larynx, PC, and CE was 47.77 Gy, 48.56 Gy, and 42.59 Gy, respectively. This may explain a lesser incidence of dysphagia in the present study.
The literature on aspiration after IMRT in HNC is sparse. Petras et al. [37], reported 34% of patients (10/29) showed aspiration on videofluorography (VFG) at 1 year after IMRT. A Dmean of 6,500 cGy or higher to the aryepiglottic folds was associated with an increased risk of aspiration at 1 year. The incidence of aspiration in the present study was 14.5% (8/55) at 1 year. We assessed aspiration based on clinical features in the majority of cases, and imaging when available. None of the patients underwent a VGF to diagnose aspiration, explaining a lower incidence of aspiration in our study.
The 3-year OS reported after IMRT in single institute series ranges from 52% to 82% [26,27]. However, the long-term survival remains poor in larger series of locally advanced HNC. The 5-year OS rate was in the range of 20%–43% for oral cancer, 8%–25% for pharyngeal cancers, and 25%–62% for laryngeal cancer with conventional treatment in a high-volume center from India [38]. The EUROCARE-5 population-based study reported 5-year age-standardized relative survival of 33.7% for all locally advanced HNC [39]. The poor OS is in concordance with other studies from India [38,40]. The tobacco use, larger gross tumor volume, and decreased sepsis surveillance might result in inferior survival. Three-fourth of our patient population were tobacco users. The smokers at diagnosis have a significantly decreased OS and were almost twice as likely to die than non-smokers [41,42]. Secondly, a larger GTV is known to have a poorer outcome. Carpen et al. [43] reported a primary GTV of >38 cm3 and a larger nodal GTV as a continuous variable had a significantly poorer OS. In the present study, the median GTV (primary + nodal) was 58.2 mL. Thirdly, the acute mortality during CRT of HNC is a poorly understood and under-reported event. Mirabile et al. [44], in a literature review, stated that the acute mortality of CRT is between 2%–9.3%, and the majority are sepsis-related. Also, the unknown causes of acute deaths are likely related to sepsis. We report 12 early deaths due to sepsis and the proposed toxicity syndrome published earlier [45]. Though OS is poor in our study, the local-regional control rate is satisfactory with medial time to LRF of 33 months.
Being a retrospective study is the main limitation. No comparison was made between the radical and adjuvant groups because of heterogenous subsites. Though different from other pharyngo-laryngeal cancers, the SCC of the salivary gland and external auditory canal were included. The primary outcome was late toxicity, and RT is similar in these patients. The strength of our study lies in excellent long-term follow-up with minimal censoring of data. The treatment received by the study population was homogenous in terms of RT dose, techniques, and chemotherapy protocol. We report rates of aspiration after IMRT and correlated with RT dose received by DARS. Aspiration is often underreported in literature due to its elusive nature.
In conclusion, we demonstrated reasonable long-term toxicities in LAHNSCC in a real-world setting. By limiting the dose to salivary glands, constrictors, and larynx, a lower incidence of xerostomia, dysphagia, and aspiration can be achieved using IMRT. Further prospective studies are needed to know the impact on quality of life after IMRT.
AcknowledgmentsThe authors thank all the staff of Department of Radiation Oncology, Medical Oncology, Surgical Oncology, Pain & Palliative, and ENT at St John’s Medical College and Hospital.
Supplementary MaterialsSupplementary materials can be found via https://doi.org/10.3857/roj.2020.00913.
Table S3.Predictors of overall survival (Cox proportional hazard analysis) Fig. 1.(A) The median time to locoregional failure was 33 months (95% confidence interval [CI], 1–76). (B) The median time to overall survival was 19.26 months (95% CI, 14.7–23.8). 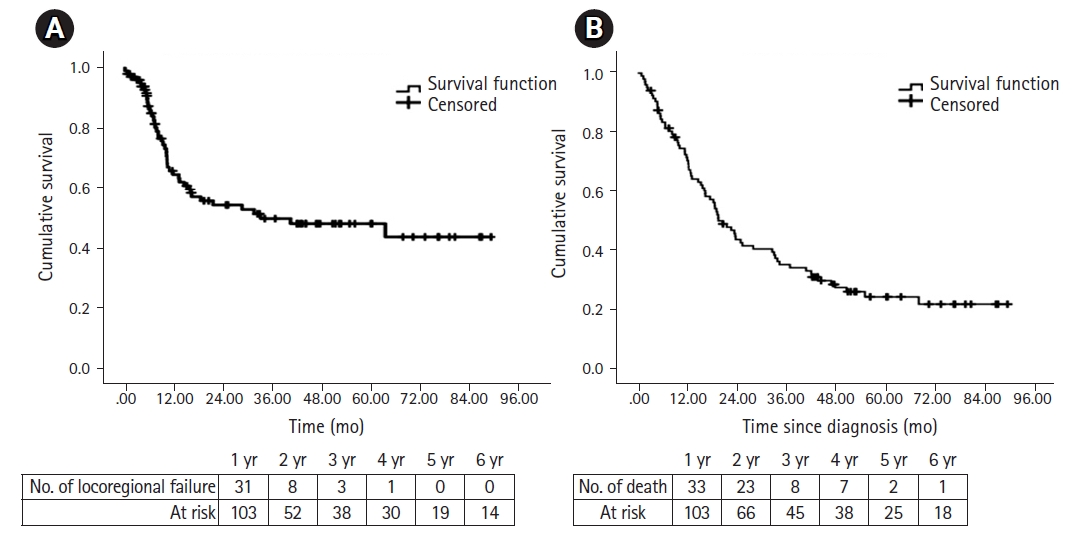
Table 1.Baseline characteristics
Cancer subsites in radical group (n = 68): hypopharynx (20), oropharynx (19), oral cavity (13), larynx (9), CUP (3), EAC (3), and SNC (1). Cancer subsites in adjuvant group (n = 35): oral cavity (26), larynx (6), CUP (1), SG (1), and SN (1). RT, radiation therapy; PORT, postoperative radiation therapy; CUP, cervical node with unknown primary; EAC, external auditory canal; SNC, sinonasal cancer; SN, sinonasal; SG, salivary gland. Table 2.Late toxicities
References1. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52.
2. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44.
3. Gregoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncol 2015;33:3277–84.
4. Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–36.
5. Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol 2012;104:343–8.
6. Marta GN, Silva V, de Andrade Carvalho H, et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol 2014;110:9–15.
7. Beadle BM, Liao KP, Giordano SH, et al. Reduced feeding tube duration with intensity-modulated radiation therapy for head and neck cancer: a Surveillance, Epidemiology, and End Results-Medicare Analysis. Cancer 2017;123:283–93.
8. Rathod S, Gupta T, Ghosh-Laskar S, Murthy V, Budrukkar A, Agarwal J. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): evidence from a prospective randomized study. Oral Oncol 2013;49:634–42.
9. Beadle BM, Liao KP, Elting LS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer 2014;120:702–10.
10. Yu JB, Soulos PR, Sharma R, et al. Patterns of care and outcomes associated with intensity-modulated radiation therapy versus conventional radiation therapy for older patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012;83:e101–7.
11. Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys 2010;76:1333–8.
12. Boero IJ, Paravati AJ, Xu B, et al. Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol 2016;34:684–90.
13. Gregoire V, Evans M, Le QT, et al. Delineation of the primary tumour clinical target volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol 2018;126:3–24.
14. Brouwer CL, Steenbakkers RJ, Bourhis J, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol 2015;117:83–90.
15. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004;60:1425–39.
16. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol 2007;85:64–73.
17. Wang X, Eisbruch A. IMRT for head and neck cancer: reducing xerostomia and dysphagia. J Radiat Res 2016;57 Suppl 1(Suppl 1):i69–i75.
18. Lauve A, Morris M, Schmidt-Ullrich R, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. II. Clinical results. Int J Radiat Oncol Biol Phys 2004;60:374–87.
19. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma: the University of Iowa experience. Int J Radiat Oncol Biol Phys 2005;63:410–21.
20. Montejo ME, Shrieve DC, Bentz BG, et al. IMRT with simultaneous integrated boost and concurrent chemotherapy for locoregionally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2011;81:e845–52.
21. Yang H, Diao LQ, Shi M, et al. Efficacy of intensity-modulated radiotherapy combined with chemotherapy or surgery in locally advanced squamous cell carcinoma of the head-and-neck. Biologics 2013;7:223–9.
22. Spiotto MT, Weichselbaum RR. Comparison of 3D confromal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PLoS One 2014;9:e94456.
23. Mazzola R, Ferrera G, Alongi F, et al. Organ sparing and clinical outcome with step-and-shoot IMRT for head and neck cancer: a mono-institutional experience. Radiol Med 2015;120:753–8.
24. Rumley CN, Nedev N, Sharples K, Lee J, Lamb DS. Intensity-modulated radiotherapy in the treatment of locoregionally advanced head and neck cancer: implementation and outcomes in a New Zealand community hospital. J Med Radiat Sci 2016;63:96–103.
25. Vlacich G, Stavas MJ, Pendyala P, Chen SC, Shyr Y, Cmelak AJ. A comparative analysis between sequential boost and integrated boost intensity-modulated radiation therapy with concurrent chemotherapy for locally-advanced head and neck cancer. Radiat Oncol 2017;12:13.
26. Fondevilla Soler A, Lopez-Guerra JL, Garcia Fernandez A, et al. Outcome and toxicity of intensity-modulated radiotherapy with simultaneous integrated boost in patients with pharyngo-laryngeal cancer. Clin Transl Oncol 2019;21:881–90.
27. Dragan T, Beauvois S, Moreau M, et al. Clinical outcome and toxicity after simultaneous integrated boost IMRT in head and neck squamous cell cancer patients. Oral Oncol 2019;98:132–40.
28. Muzumder S, Srikantia N, Udayashankar AH, Kainthaje PB, John Sebastian MG. Burden of acute toxicities in head-and-neck radiation therapy: a single-institutional experience. South Asian J Cancer 2019;8:120–3.
29. Muzumder S, Nirmala S, Avinash HU, Sebastian MJ, Kainthaje PB. Analgesic and opioid use in pain associated with head-and-neck radiation therapy. Indian J Palliat Care 2018;24:176–8.
30. Muzumder S, Nirmala S, Avinash HU, Kainthaje PB, Sebastian MJ, Raj JM. Early competing deaths in locally advanced head-and-neck cancer. Indian J Palliat Care 2018;24:446–50.
31. Muzumder S, Srikantia N, Vashishta GD, et al. Compliance, toxicity and efficacy in weekly versus 3-weekly cisplatin concurrent chemoradiation in locally advanced head and neck cancer. J Radiother Pract 2019;18:21–5.
32. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.
33. Clifford Chao KS. Practical essentials of intensity modulated radiation therapy. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
34. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03 [Internet]. Bethesda, MD: National Cancer Institute; c2021 [cited 2021 Aug 27]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/About.html.
35. Baudelet M, Van den Steen L, Tomassen P, et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck 2019;41:3594–603.
36. De Felice F, de Vincentiis M, Luzzi V, et al. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral Oncol 2018;77:125–30.
37. Petras KG, Rademaker AW, Refaat T, et al. Dose-volume relationship for laryngeal substructures and aspiration in patients with locally advanced head-and-neck cancer. Radiat Oncol 2019;14:49.
38. Rao DN, Shroff PD, Chattopadhyay G, Dinshaw KA. Survival analysis of 5595 head and neck cancers: results of conventional treatment in a high-risk population. Br J Cancer 1998;77:1514–8.
39. Gatta G, Botta L, Sanchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer 2015;51:2130–43.
40. Nandakumar A, Nandakumar A. Survival in head and neck cancers: results of a multi- institution study. Asian Pac J Cancer Prev 2016;17:1745–54.
41. Farshadpour F, Kranenborg H, Calkoen EV, et al. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck 2011;33:817–23.
42. Osazuwa-Peters N, Adjei Boakye E, Chen BY, Tobo BB, Varvares MA. Association between head and neck squamous cell carcinoma survival, smoking at diagnosis, and marital status. JAMA Otolaryngol Head Neck Surg 2018;144:43–50.
43. Carpen T, Saarilahti K, Haglund C, et al. Tumor volume as a prognostic marker in p16-positive and p16-negative oropharyngeal cancer patients treated with definitive intensity-modulated radiotherapy. Strahlenther Onkol 2018;194:759–70.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |