Immunomodulatory effect of splenectomy in lung cancer mouse xenograft models receiving radiation therapy
Article information
Abstract
Purpose
This study aims to investigate the effect of splenectomy on radiation-mediated growth inhibition and immune modulation in lung cancer xenograft models.
Materials and Methods
Human non-small cell lung cancer H1299 cells and murine Lewis lung carcinoma LL/2-luc cells were injected into the right hind leg of BALB/c-nude mice and C57BL/6 mice, respectively. Splenectomy or sham operation was performed prior to tumor cell injection or before and after irradiation during tumor growth. Irradiation was delivered with 2–3 fractions of 6 Gy X-ray using a linear accelerator. Flow cytometry analysis was performed for immune cell profiling.
Results
Splenectomy prior to tumor injection or at early stage inhibited growth of LL/2-luc tumors but not that of H1299 tumors; however, it did not enhance the antitumor effect of radiation regardless of intervention timing. Flow cytometry analysis showed monocytic myeloid-derived suppressor cells (MDSCs) and activated CD8+ T cells increased after irradiation in the tumors of splenectomized mice, compared to those of sham-operated mice. Administration of anti-PD-1 (programmed death-1) antibodies improved the ability of splenectomy to attenuate the growth of irradiated tumors.
Conclusion
Splenectomy has paradoxical effects on radiation-induced tumor growth inhibition, depending on tumor types and intervention timing, but it has an immune-modulating effect when combined with radiation.
Introduction
Radiation therapy (RT), a major local treatment modality for cancer, exerts an array of immune modulatory effects [1-3]. Myeloid-derived suppressor cells (MDSCs) are suggested to be one of the RT-induced immune suppression mechanisms, thereby contributing to radioresistance [4,5]. MDSCs are a heterogeneous group of immature myeloid lineage, and two major subsets of MDSCs have been identified: polymorphonuclear (PMN)-MDSCs and monocytic (M)-MDSCs. M-MDSCs differentiate into mature macrophages (tumor-associated macrophage [TAM]) and dendritic cells, and PMN-MDSCs share phenotypical characteristics with neutrophils (tumor-associated neutrophil [TAN]), respectively [6]. TANs have been classified as anti-tumor N1 TANs and pro-tumor N2 TANs, and TANs, likely N2 type, have been associated with the poor prognosis in many cancers [7-9]. Therefore, MDSCs or TANs can be therapeutic targets to overcome the resistance against cancer treatments including RT [10-12].
The spleen, the largest secondary lymphoid organ, is a major site of extramedullary hematopoiesis and of MDSCs accumulation in cancers [13]. Thus, splenectomy, a physical removal of the spleen, has been investigated as a therapeutic option to deplete MDSCs and enhance tumor control [5,14,15], but the effects of splenectomy on tumor growth in mouse models are controversial and context-dependent [14-19]. Even though MDSCs modify efficacy of RT [12,20], it remains unclear whether splenectomy has additional effects on RT. The goal of this study is to explore the effect of splenectomy on radiation-mediated tumor growth inhibition and immune modulation. We hypothesized that splenectomy leads to a decrease in tumor-infiltrating MDSCs, resulting in enhancement of RT effect. To prove this, we determined the effect of splenectomy on the growth of lung cancer xenografts receiving RT and on the distribution of immune cells, including MDSCs and T lymphocytes by performing immunophenotyping. Then, we further investigated several strategies for optimizing splenectomy to enhance the effect of RT.
Materials and Methods
1. Cell lines
Murine lung carcinoma LL/2-Red-FLuc (LL/2-luc) cells were obtained from PerkinElmer (Waltham, MA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 g/mL streptomycin, 2 mM L-glutamine, and 25 mM HEPES. Cultures were maintained in a humidified atmosphere of 95% air/5% CO2 at 37°C. Human non-small cell lung cancer (NSCLC) H1299 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS, 100 U/mL penicillin, 100 g/mL streptomycin, 2 mM L-glutamine, and 25 mM HEPES.
2. Animal models
All animal experiments were performed and approved in accordance with the Institutional Animal Care and Use Committee (IACUC) of Samsung Biomedical Research Institute (No. 20180625002, Approval date 2018-07-03; No. 20190926001, Approval date 2019-10-18). The animal study was carried out in compliance with the ARRIVE guidelines [21]. Male BALB/c-nude or C57BL/6 mice, 4–5 weeks old, were purchased from Orient Bio (Gapyeong, Korea).
In BALB/c-nude mice, splenectomy or sham operation was performed prior to tumor cell injection. H1299 cells were harvested and suspended in a 1:1 ratio of phosphate-buffered saline (PBS) and Matrigel (Corning Inc., Tewksbury, MA, USA), and 1 × 106 cells/20 µL cells were injected subcutaneously into the right hind leg. Tumor volumes were calculated every 3 days with calipers according to the following formula:
When the mean tumor volume reached 500 mm3, tumors were irradiated with 2 fractions of 6 Gy X-ray on consecutive days (total dose of 12 Gy) to the right hind leg.
A LL/2-luc tumor model was generated by subcutaneously injecting LL/2-luc cells (1 × 106 cells/20 µL in 50% Matrigel) into the right hind leg in C57BL/6 mice. Splenectomy or sham operation was performed prior to tumor injection, at an early stage of tumor development (mean tumor volume of 200 mm3), or at an advanced stage of tumor growth (mean tumor volume of 1,000 mm3). Tumor irradiation began from the 7th day after tumor injection. Irradiation was given as 3 fractions of 6 Gy X-ray on consecutive days (total dose of 18 Gy). Mice in the treatment groups were intraperitoneally treated with anti-PD-1 (programmed death-1) antibody (2.5 mg/kg; Bio X Cell, Lebanon, NH, USA) on days 7, 9, 12, and 16.
3. Irradiation experiments
Tumors implanted into mouse hind legs were irradiated as previously described [22]. Briefly, 6 MV photon beams were delivered at a dose rate of 3.96 Gy/min using a Varian Clinac 6EX linear accelerator (Varian Medical System, Palo Alto, CA, USA). Prior to irradiation, mice were fixed by anesthetizing with intraperitoneal injections of 30 mg/kg zolazepam/tiletamine and 10 mg/kg xylazine. Tumor-beading hind legs were placed inside the radiation field (30 cm × 7 cm) and covered by a 2-cm-thick water-equivalent bolus with a source-to-surface distance of 100 cm. The TG-51 protocol was used for calibration of the absolute dose.
4. Splenectomy and sham surgeries
Splenectomy or sham operation was performed under anesthesia induced by a sufficient amount of isoflurane using a liquid chamber for the vaporizer. Splenectomy was performed via a 0.5-cm skin incision made in the lateral abdomen and a midline fascia incision of the same length. The spleen was separated from the stomach and removed outside of the incision. The midline fascial incision was sutured with 4–0 thread and the skin incision was closed using 6–0 thread. The sham operation was performed by a skin incision and a midline fascia incision, in the same way as the splenectomy. However, the spleen remained inside the incision wound and the incision was closed. When surgeries were completed, mice were placed under a heat lamp and antibiotics (Baytril 50 inj., 5 mg/kg; Bayer, Leverkusen, Germany) and antiphlogistic drugs (Metacam 2%, 1 mg/kg; Boehringer Ingelheim, Ingelheim am Rhein, Germany) were injected intramuscularly for 3 days.
5. Flow cytometry analysis
Tumors were harvested from the right hind leg, cut into small pieces, minced into a single-cell suspension, and mashed through a 70-μm cell strainer. Red blood cells were lysed with red blood cell lysing buffer (BD Bioscience, Franklin Lakes, NJ, USA).
Cell suspensions were stained with fluorescence-conjugated antibodies specific for CD45, CD11b, Ly6G, Ly6C, CD3, CD4, CD8, CD25 (BD Bioscience), and PD-L1 (eBioscience Inc., San Diego, CA, USA). For intracellular staining, cells were fixed with Fixation/Permeabilization buffer (eBioscience Inc.) and stained with anti-Foxp3 and anti-IFNγ (BD Bioscience) antibodies. Stained cells were analyzed by flow cytometry using a BD FACSVerse (BD Bioscience) and data analysis was performed using FlowJo software (BD Bioscience).
6. Immunohistochemistry
Tumors harvested were placed immediately into 10% neutral-buffered formalin (NBF). Tissues embedded in paraffin were sectioned at 4 μm. The tissue sections were stained with hematoxylin and eosin (H&E) for routine histological evaluation, and immunohistochemistry (IHC) was performed using the primary antibodies against CD3 (1:100 dilution), CD4 (1:1000 dilution), CD8 (1:2000 dilution; Abcam, Cambridge, UK) and Gr-1 (Ly6G/Ly6C, 1:100 dilution; Bio X Cell). Slides were digitally scanned with a digital pathology scanning system (Aperio ScanScope XT; Leica Biosystems, Buffalo Grove, IL, USA) and analyzed by using the ImageScope software (Leica Biosystems).
7. Statistics
Statistical analyses were performed using GraphPad Prism 7 (Graphpad Software, San Diego, CA, USA). The significance of differences between experimental groups was calculated using one-way analysis of variance with Bonferroni’s test. The p-values <0.05 were considered statistically significant.
Results
1. Radiation modulates splenic immune cells in syngeneic mice harboring LL/2 tumors
To understand a role of spleen, the largest lymphatic organ, in anti-tumor activity of radiation, LL/2 tumor-bearing legs were irradiated with a single fraction of 12 Gy or three fractions of 6 Gy (total 18 Gy), and spleens were collected either a day or 15 days after irradiation (Fig. 1A). Radiation significantly inhibited tumor growth (p < 0.001), but little difference between two irradiation conditions was observed (Fig. 1B). The immunophenotyping results showed that splenic neutrophils (Ly6G+CD11b+), M-MDSCs (CD45+CD11b+Ly6G-Ly6C+) and PMN-MDSCs (CD45+CD11b+Ly6G+Ly6Clow) were significantly reduced by tumor irradiation 15 days after irradiation but not a day after irradiation (Fig. 2A). Regarding T cells, three fractions of 6 Gy but not a single 12 Gy significantly reduced splenic CD8+ T cells and increased exhausted PD1+CD8+ T cells one day after irradiation (Fig. 2B). In contrast, populations of CD4+ and CD8+ T cells along with exhausted CD8+ T cells significantly increased in the spleens from mice received three fractions of 6 Gy (Fig. 2B). Regulatory T cells (CD4+CD25+Foxp3+; Treg) increased only in the spleens of mice received a single 12 Gy of X-ray. These data suggest local radiation to tumor sites modulates distribution of immune cells residing in spleens.

Radiation inhibits growth of LL/2-luc tumors in a syngenic mouse model. (A) Experimental scheme. Murine Lewis lung carcinoma LL/2 cells were implanted into hind legs of C57BL/6 mice. The tumors were irradiated with either a single fraction of 12 Gy or 3 fractions of 6 Gy of X-rays. Splenectomy was performed one day or 15 days after irradiation (IR). (B) Tumor growth curves of LL/2 in mice. Radiation significantly reduced tumor growth, but there was little difference between two irradiation conditions. Data are presented as the mean ± standard error of the mean (n ≥ 4). All statistical analysis was performed using one-way analysis of variations (ANOVA) with a Bonferroni’s multiple comparisons test at the 5% level. ***p < 0.001, ****p < 0.0001.
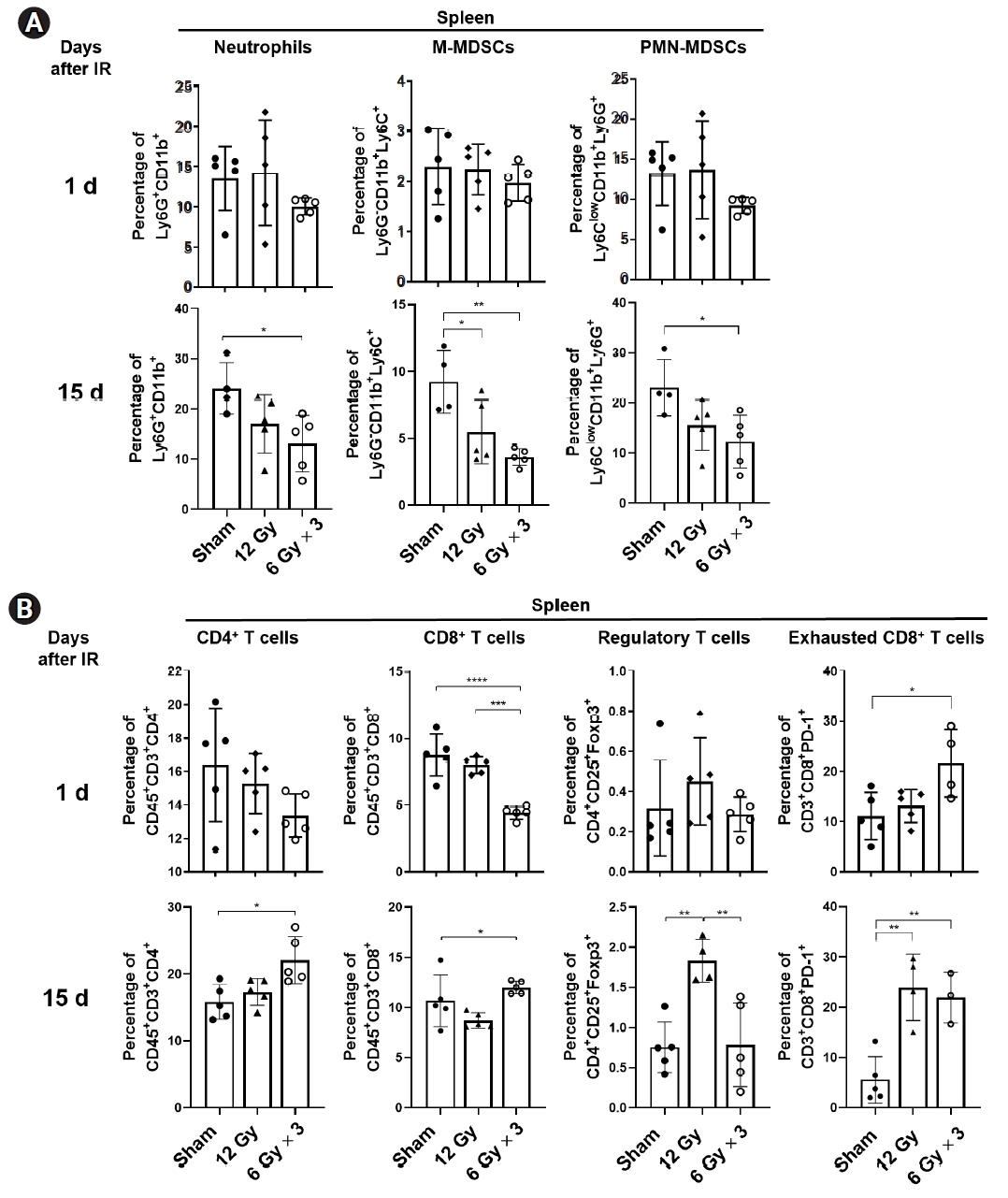
Radiation modulates distribution of immune cells within the spleen of mice harboring LL/2-luc tumors. (A) Flow cytomtry analysis of neutrophils, M-MDSC, and PMN-MDSC in the spleens harvested one day or 15 days after irradiation (IR) to tumor sites. (B) Flow cytometry analysis of T lymphocytes in the spleens. Percentage of splenic CD4+ T cells, CD8+ T cells, regulatory T cells, and exhausted T cells was analyzed. Data are presented as the mean ± standard error of the mean (n ≥ 4). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. MDSC, myeloid-derived suppressor cells; M, monocytic; PMN, polymorphonuclear.
2. Splenectomy inhibits tumor growth but does not enhance the antitumor effect of radiotherapy in a syngeneic LL/2 model
To evaluate how splenectomy affects radiation-induced tumor growth delay in a syngeneic LL/2 tumor model, splenectomy in C57BL/6 mice was performed prior to tumor injection (Fig. 3A). Tumor-bearing legs were irradiated with three fractions of 6 Gy because this treatment regimen was more effective than a single 12 Gy in terms of modulation of splenic immune cells (Fig. 2A, 2B). In the LL/2-luc mouse model, tumor growth was significantly delayed by either irradiation (42.3% tumor growth inhibition; p = 0.0056) or splenectomy (46% growth inhibition; p = 0.0062). The combination treatment did not show any additive effect (Fig. 3B, 3C). These results suggest that splenectomy prior to tumor injection inhibits tumor growth but does not enhance the anti-tumor effect of RT in the syngeneic LL/2 tumor model.
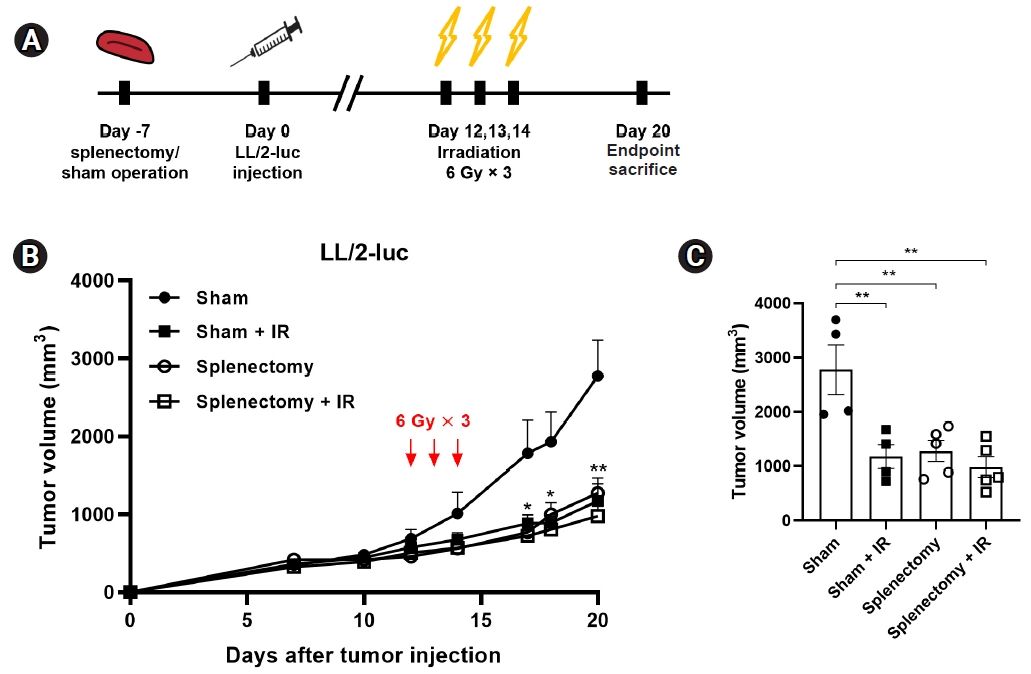
Splenectomy prior to tumor injection delayed tumor growth in a syngeneic LL/2-luc tumor model. (A) Experimental scheme. Splenectomy or sham operation was performed prior to tumor injection in C57BL/6 mice. The tumor-bearing mice were irradiated with 3 fractions of 6 Gy (tumor volume of 500 mm3). (B) Tumor growth curves of murine LL/2-luc cells in mice and (C) tumor volume at day 20 after tumor inoculation. In the splenectomy group, the tumor volume was decreased on day 20. Data are presented as the mean ± standard error of the mean (n ≥ 4). IR, irradiation. *p < 0.05, **p < 0.01.
Flow cytometric analyses revealed that there was no difference in the populations of neutrophils and M-MDSCs between splenectomized mice and sham-operated mice (upper panels in Fig. 4A). A trend towards an increase in PMN-MDSC populations was seen by irradiation (p = 0.3605) and a significant increase was seen with splenectomy (p = 0.0361; right upper in Fig. 4). M-MDSC populations significantly increased in the combination group compared to the irradiation group (p = 0.0045) (middle upper in Fig. 4A). We also observed an increase in CD3+, CD8+, and CD4+ T cell populations in both the radiation and splenectomy groups compared to the sham group (upper panel in Fig. 4B). In the combination group, compared to the irradiation group, IFNγ-releasing activated CD8+ T cells (IFNγ+CD8+) significantly increased (27.8% vs. 43.6%; p = 0.001) (lower left in Fig. 4B). The Treg cells increased after irradiation but decreased after the combination treatment (1.56% vs. 0.92%; p = 0.1512) (lower right in Fig. 4B).
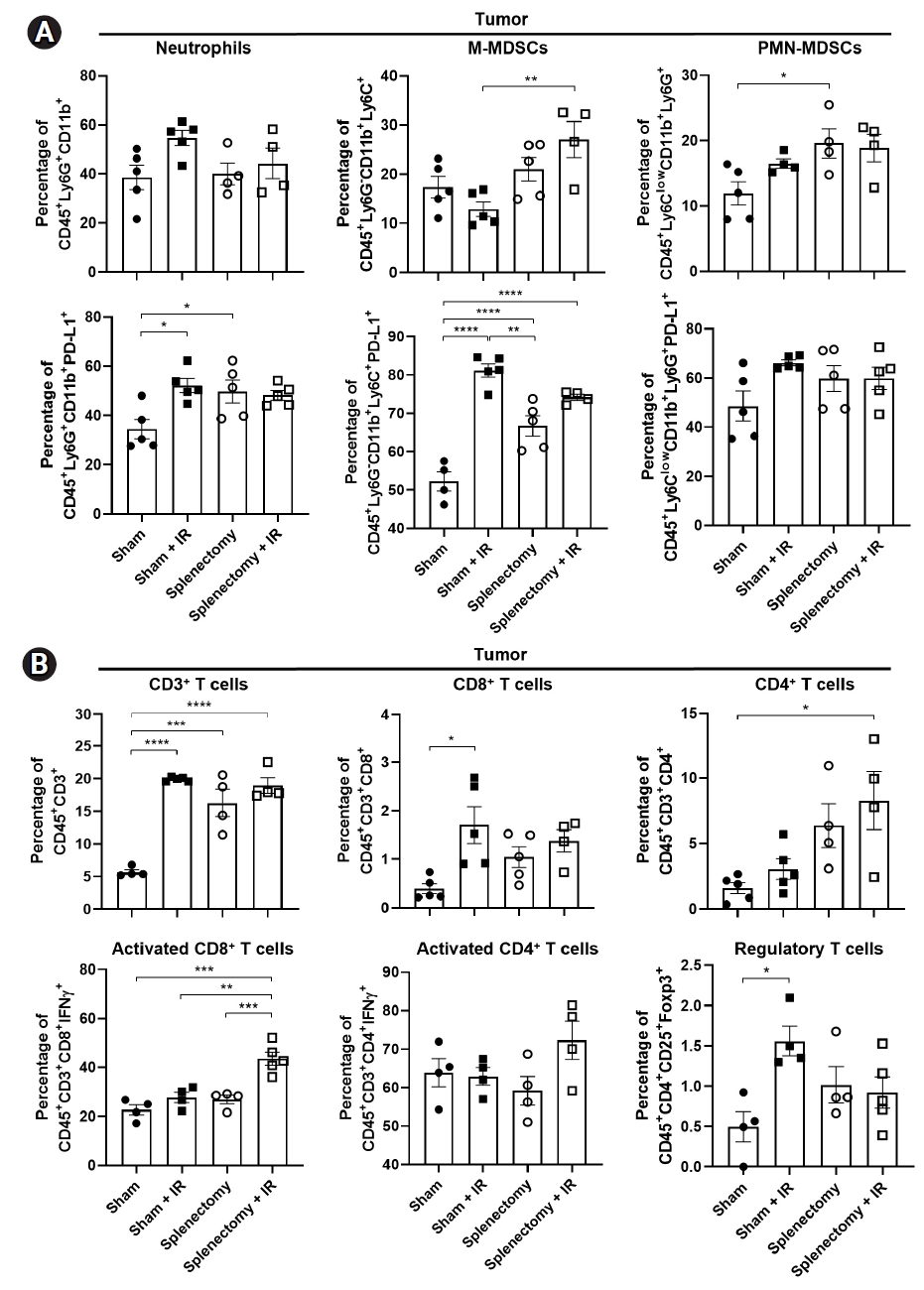
Splenectomy enhanced the activation of tumor-infiltrating T lymphocytes in a LL/2-luc tumor model. Flow cytometry analysis of tumor-infiltrating (A) MDSCs and (B) T lymphocytes in a LL/2-luc tumor model. (A) M-MDSCs were increased in splenectomized mice with fractionated irradiation (IR). The population of PD-L1+ cells was also increased by irradiation. (B) IFNγ-releasing active CD8+ T cells were increased and Treg were decreased in splenectomized mice when combined with IR. Data are presented as the mean ± standard error of the mean (n = 4). MDSC, myeloid-derived suppressor cell; M, monocytic; PMN, polymorphonuclear; PD-L1, programmed death-ligand 1; INF, interferon. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. Splenectomy has limited antitumor effect in a human H1299 lung cancer xenograft model
To evaluate the effect of splenectomy on human lung tumor growth in immune-compromised mice, human NSCLC H1299 cells were implanted into hind legs of splenectomized BALB/c nude mice. H1299-tumor-bearing hind legs were irradiated with a total of 12 Gy of X-rays in two fractions (Fig. 5A). As expected, irradiation attenuated tumor growth significantly (sham vs. sham + IR: 41.7% reduction, p = 0.0006; splenectomy vs. splenectomy + IR, 35.2% reduction, p = 0.0014). Although not statistically significant, there was a trend that splenectomy prior to tumor injection slightly reduced tumor growth in the H1299 xenograft mouse model (Fig. 5B, 5C).

Splenectomy prior to tumor injection had the limited effect on tumor growth in a human H1299 xenograft model. (A) Experimental scheme. Splenectomy or sham operation was performed prior to tumor injection in BALB/c-nude mice. H1299 tumor-bearing mice were irradiated with 2 fractions of 6 Gy (tumor volume of 500 mm3). (B) Tumor growth curves of H1299 in mice and (C) tumor volume at day 45 after tumor inoculation. Fractionated irradiation (IR) delayed tumor growth. Flow cytometry analysis of (D) neutrophils, (E) M-MDSC, and (F) PMN-MDSC frequency in tumors. M-MDSC are increased in splenectomized mice with fractionated IR. The percentage of PD-L1+ cells are also presented. Data are presented as the mean ± standard error of the mean (n ≥ 4). MDSC, myeloid-derived suppressor cell; M, monocytic; PMN, polymorphonuclear; PD-L1, programmed death-ligand 1. *p < 0.05, **p < 0.01, ****p < 0.0001.
Populations of neutrophils and PMN-MDSCs were not changed by radiation, splenectomy, or combination (Fig. 5D, 5F). The M-MDSC population significantly increased in the combination group (sham + irradiation vs. splenectomy + irradiation, p < 0.0001) (Fig. 5E). Irradiation had a tendency to slightly increase populations of myeloid cells, such as MDSCs and neutrophils, although no statistical significance was seen. Expression of PD-L1 on M-MDSCs was also induced in the combination group, compared to splenectomy group (Fig. 5E).
4. Splenectomy decreases infiltration of Gr-1 positive cells to tumors received radiation in both LL/2 and H1299 tumor models
IHC analysis of Gr-1 (also Ly6C/Ly6G) showed that splenectomy significantly decreased Gr-1 positive cells in H1299 tumors with or without radiation (Fig. 6A, 6B). In a LL/2-luc syngeneic tumor model, radiation markedly increased Gr-1 positive cells (p < 0.01), which was suppressed by splenectomy (p < 0.05) (Fig. 6C, 6D). Radiation increased infiltration of CD3+ and CD8+ cells to LL/2-luc tumors (Fig. 6C, 6D), which is consistent with the flow cytometric immunophenotyping results (Fig. 4B).

Splenectomy modulated the infiltration of immune cells to tumor in H1299 and LL/2-luc tumor models. (A) Representative immunohistochemistry (IHC) images of Gr-1 in H1299 tumor tissues and (B) quantification. The mice bearing H1299 tumors were treated as described in Fig. 5A. (C) Representative IHC images of CD3, CD8, CD4 and Gr-1 in LL/2-luc tumor tissues and (D) quantification. The mice bearing LL/2-luc tumors were treated as described in Fig. 3A. Data are presented as the mean ± standard error of the mean (n = 15). IR, irradiation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
5. Antitumor effect of splenectomy depends on its timing in the syngeneic lung cancer model
Splenectomy was performed at two different time points after the injection of LL/2-luc tumors in C57BL/6 mice; at an early stage of tumor growth (mean tumor volume of 200 mm3), or at an advanced stage (mean tumor volume of 1,000 mm3) (Fig. 7A). As shown in previous experiments, fractionated irradiation significantly inhibited tumor growth in all groups (Fig. 7B, 7C). We observed that splenectomy at the advanced stage had no inhibitory effect on tumor growth compared with sham operation on day 20 after tumor injection (sham vs. 2nd splenectomy, 2,291.9 mm3 vs. 2,034.2 mm3). In contrast, splenectomy at the early stage significantly suppressed tumor growth (sham vs. 1st splenectomy, 2,291.9 mm3 vs. 1,387.6 mm3; p = 0.0349) (Fig. 7B, 7C). However, splenectomy after tumor injection did not enhance radiation-induced tumor growth delay regardless of timing of splenectomy relative to radiation.
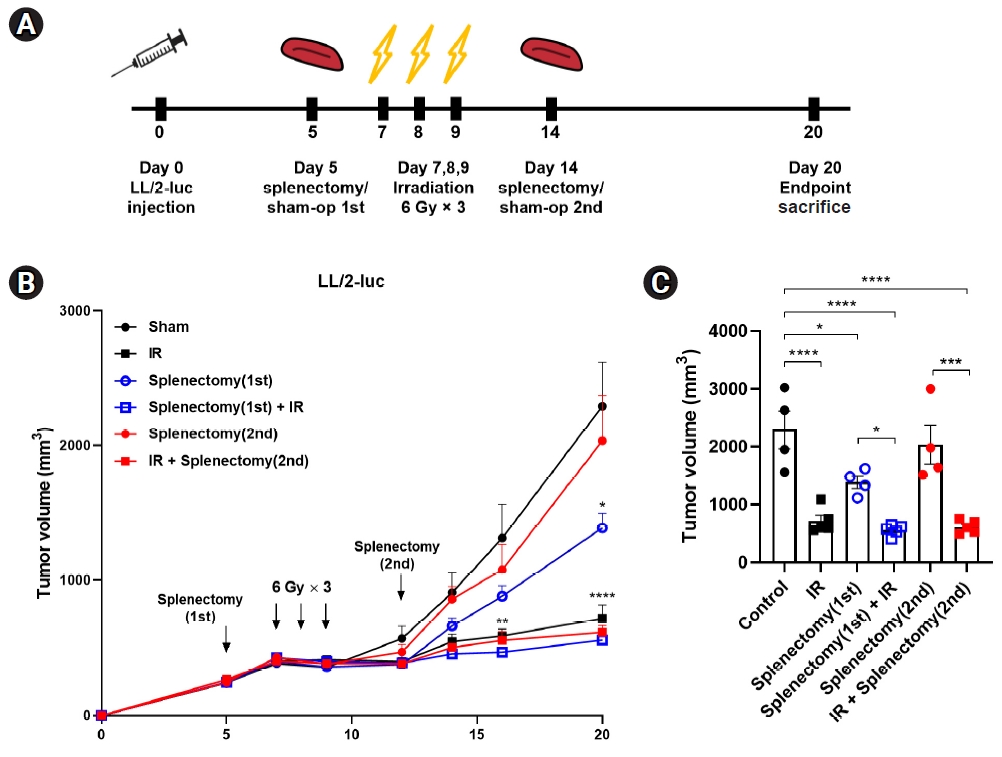
Splenectomy performed at an early stage of tumor growth had a tendency to inhibit tumor growth. (A) Experimental scheme. Splenectomy was performed at an early stage of tumor growth (tumor volume of 200 mm3) before irradiation (IR) or at an advanced stage (tumor volume of 1,000 mm3) after irradiation. (B) Tumor growth curves of LL/2-luc in mice and (C) tumor volume at day 20 after tumor inoculation. Splenectomy at an early stage (1st) but not that at the advanced stage (2nd) had a significant inhibitory effect on tumor growth. Data are presented as the mean ± standard error of the mean (n ≥ 4). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
On the basis of the results that the combination of splenectomy and radiation increased tumor infiltration of PD-L1+ MDSCs (Fig. 4A), we further examined the effect of anti-PD-L1 antibodies on growth of tumors in mice received radiation or splenectomy (Fig. 8A). Administration of anti-PD-L1 antibodies led to a significant delay in LL/2-luc tumor growth (Fig. 8B, 8C). Radiation alone decreased tumor growth, which was not enhanced by splenectomy as shown in Fig. 8B. However, triple combination of radiation, anti-PD-1, and splenectomy showed the strongest inhibitory effect on LL/2 tumor growth (Fig. 8B, 8C).

Administration of anti-PD-1 antibodies enhanced antitumor activity of IR in splenectomized mice. (A) Experimental scheme. Splenectomy was performed at an early stage of tumor growth. The LL/2-luc-tumor-bearing mice were irradiated with 3 fractions of 6 Gy and treated with an anti-PD-1 antibody on day 7, 9, 12, and 16. (B) Tumor growth curves of LL/2-luc in mice and (C) tumor volume at day 23 after tumor inoculation. Data are presented as the mean ± standard error of the mean (n ≥ 4). PD-1, programmed death-1; IR, irradiation. *p < 0.05, **p < 0.01, ****p < 0.0001.
Discussion
In the present study, we aimed to investigate the effect of splenectomy on radiation-mediated tumor growth inhibition and immune modulation in a variety of settings. Radiation significantly decreased tumor burden in the syngeneic mouse model of lung cancer (Fig. 1). Immunophenotyping results of splenic immune cells after tumor irradiation showed that radiation-induced tumor growth inhibition affected immune cell populations in the spleen (Fig. 2). CD4+ and CD8+ T cells but not MDSCs decreased in the spleen excised a day after irradiation, which may be related to sensitivity to irradiation. After 15 days, decreased tumor burden may reduce proliferation of splenic MDSCs and increased that of splenic CD4+ and CD8+ T cells (Fig. 2A, 2B). An increase in PD-1+ CD8+ T cells may be related to T cell exhaustion.
Our findings using splenectomized mice revealed that the anti-tumor effect of splenectomy is complex and context-dependent, as previously described by Prehn [16]. Firstly, splenectomy prior to tumor injection exerted a significant anti-tumor effect in immune-competent mice (Fig. 3) but not in immune-compromised mice (Fig. 5), suggesting a potential role of T cells in splenectomized mice. Splenectomy showed an additional inhibitory effect on mice who underwent irradiation, although the difference was not statistically significant. The anti-tumor effect of splenectomy after tumor injection depended on the timing relative to radiation (Fig. 7). Mice receiving splenectomy prior to irradiation had smaller tumors compared to mice that were splenectomized after irradiation.
Immunophenotyping showed that intratumoral M-MDSCs increased after combination treatment with irradiation and splenectomy in both LL/2-luc and H1299 xenograft tumor models, although the anti-tumor effect of splenectomy in the two experiments was different (Figs. 3, 5). Furthermore, there was a tendency of increased PD-L1 expression in neutrophils, M-MDSCs, and PMN-MDSCs after irradiation or splenectomy, compared to that in sham control, in the immune-competent mouse model (Fig. 4). An increase in intratumoral MDSCs and PD-L1 expression following irradiation is one of the well-known immunomodulatory effects of local irradiation [23-25], and our results are consistent with these phenomena. Combined treatment of splenectomy and irradiation increased the population of IFNγ-expressing active CD8+ T cells with a decrease in Treg cells (Fig. 4), which may lead to the strong inhibition of LL/2-luc tumor growth.
A previous study using murine NSCLC cells by Levy et al. [15] demonstrated that splenectomy in immune-compromised mice did not show anti-tumor activity, which is consistent with our results (Fig. 1). The study also showed that in several syngeneic models, splenectomy performed at an advanced stage of tumor growth (mean tumor volume of 500 mm3), but not prior to tumor injection or at an early stage of tumors growth, had a strong inhibitory effect on tumor growth with reduced circulating and infiltrating M-MDSCs. Unlike the previous data, our results showed that tumor infiltrating MDSCs were not reduced by splenectomy, and rather, they were increased by a combination of radiation and splenectomy, suggesting that bone marrow but not spleen may be a major supplier in our experimental settings. However, IHC staining of Gr-1 showed that splenectomy suppressed infiltration of Gr-1-positive myeloid cells into H1299 and LL/2-luc tumors received radiation, without affecting CD3/CD4/CD8 cells (Fig. 8). Thus, more sophisticated analysis on MDSC subsets may be necessary. Targeting MDSC recruitment is a way to improve the efficacy of RT in various preclinical models [5,26,27]. Colony stimulating factor 1 receptor (CSF1R) blockade has been shown to inhibit radiation-induced MDSC infiltration in prostate cancer [26]. The knockout of C-C chemokine receptor type 2 (CCR2) or administration of anti-CCR2 antibodies has also resulted in the inhibition of radiation-induced MDSC infiltration [27]. Mabuchi et al. [5] showed that granulocyte colony-stimulating factor (G-CSF)high tumor-bearing mice exhibited splenomegaly and radioresistance compared to G-CSFlow tumor-bearing mice. MDSC depletion with an anti-Gr-1 antibody or splenectomy has also been shown to enhance the efficacy of RT against G-CSFhigh tumors but not G-CSFlow tumors. These data, as well as ours, suggest the effect of splenectomy could be maximized for RT in a situation when the spleen serves as a major reservoir of MDSCs. However, the splenectomy could not be widely applicable in clinical setting. Then, non-invasive approach targeting MDSC should be considered.
Synergistic antitumor effect of combined RT and anti-PD-L1 treatment has been well known, and reduced accumulation of MDSCs are also observed following this combination [23,28]. As combined RT and immune checkpoint inhibitors has been incorporated into treatment of locally advanced NSCLC [29,30], therapeutic approach to enhance the efficacy of this combination is needed in future directions. A growing body of evidence suggests MDSCs as a predictive marker in immune checkpoint inhibitors [31-33], and a variety of strategies targeting MDSCs, including histone deacetylase (HDAC) inhibitors, have been applied to enhance the effect of anti-PD-1 or PD-L1 treatment [34-37]. A recent study by Lan et al. [38] showed that ablative hypofractionated RT (AHFRT) reduced the recruitment of MDSC by inhibiting VEGF signaling, resulting in greater efficacy in tumor control when combined with anti-PD-L1, compared to conventional fractionated RT (CFRT). The total radiation dose used for AHFRT in that study was 23 Gy in 2 fractions, which is higher than ours (18 Gy in 3 fractions), possibly leading to the opposite effect on the PD-L1+MDSC population. As there was an increase in the populations of intratumoral PD-L1+MDSCs and splenic PD-1+ CD8+ T cells in the radiation groups, targeting PD-1/PD-L1 signaling boosted anti-tumor effect of radiation in our experimental settings.
Our study has several limitations. The number of mice used was small, and flow cytometric analysis was performed at a single time point. Serial measurements of immune cell populations in more tumors at different time points might have given us more information regarding immunomodulatory effect of RT with or without splenectomy. Total dose of 18 Gy significantly attenuated tumor growth in all experimental conditions, which may make addition of splenectomy less effective. Thus, a further study is warranted to test the radiation dose-dependent effect on splenectomy.
Taken together, our data demonstrate that anti-tumor effect of splenectomy in tumor-bearing mice treated with radiation may be complex, depending on tumor types and intervention timing in lung cancer mouse models. These may be due to the extreme complexity of tumor-immune cell interactions within the microenvironment of different experimental settings. Our findings provide insight into the mechanism of immunomodulatory function of splenectomy during RT, such as an increase in activated T cells and PD-L1+ MDSC in tumors. Thus, our future studies will be directed to assess different non-invasive approaches to modulate the immunosuppressive effect of MDSCs and find optimal strategies of MDSC modulation to enhance radiotherapeutic or radio-immunotherapeutic effects.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (No. NRF-2017M2A2A7A02018569, NRF-2019R1F1A1061950).
