Long-term oncological outcomes of hypofractionated versus conventional fractionated whole breast irradiation with simultaneous integrated boost in early-stage breast cancer
Article information
Abstract
Purpose
For patients with early breast cancer who undergo breast-conserving surgery, adjuvant whole breast irradiation (WBI) with simultaneous integrated boost (SIB) results in lower radiotherapy fractions. Published studies have shown that both conventional fraction with SIB (C-SIB) and hypofractionation with SIB (H-SIB) seem to be safe and feasible. In this study, we sought to compare the oncologic outcomes between C-SIB and H-SIB in early-stage breast cancer.
Materials and Methods
Stage I–II breast cancer patients who received adjuvant WBI with SIB between January 2008 and December 2017 were retrospectively reviewed. The radiation dose in the C-SIB group was 50 Gy and 65 Gy in 25 daily fractions, while in the H-SIB group, it was 43.2 Gy and 52.8 Gy in 16 daily fractions to the whole breast and tumor bed, respectively.
Results
A total of 188 patients, 103 in the C-SIB group and 85 in the H-SIB group, were included. With a median follow-up time of 87 months, 7-year locoregional control of C-SIB was comparable to H-SIB (95.8% vs. 97.4%, p = 0.964). The 7-year distant metastasis-free survival rates of C-SIB and H-SIB were 89.9% and 95.9% (p = 0.111), while the 7-year disease-free survival rates were 84.2% and 95.4%, respectively (p = 0.176). In multivariate analysis, there was no significant prognostic factor associated with better overall survival.
Conclusion
H-SIB provided comparable locoregional control to C-SIB. With the advantage of a shorter radiotherapy course, H-SIB could be a favorable option for WBI in early-stage breast cancer.
Introduction
Breast cancer is the most common cancer among women worldwide. The number of new cases in 2020 was approximately 2.3 million, with 684,996 deaths [1]. In Thailand, the mean annual age standardized incidence rate per 100,000 women diagnosed with breast cancer was 31.36 [2]. For early-stage breast cancer, breast-conserving surgery (BCS) followed by postoperative radiotherapy is one of the standard treatments and has oncologic outcomes comparable to those of mastectomy [3-5]. Generally, postoperative radiotherapy after BCS consists of whole breast irradiation (WBI) with or without a tumor bed boost [6]. The recommended conventional radiation dose of WBI is 45–50 Gy in 25 daily fractions followed by a sequential tumor bed boost of 10–16 Gy in 5–8 daily fractions, resulting in a radiotherapy treatment course of up to 7 weeks [7].
To decrease the treatment course, hypofractionated WBI has been accepted as a standard treatment in early breast cancer with oncologic and cosmetic outcomes comparable to those of conventional fractionation [8-10]. The dose of moderate hypofractionated WBI varied from 40–42.5 Gy in 13–16 daily fractions, which could shorten the treatment course from approximately 5 to 3 weeks. However, sequential tumor bed boost was still administered in a number of centers, which eventually prolonged the overall treatment course.
In recent decades, there has been growing interest in the delivery of a simultaneous integrated boost (SIB) to the tumor bed during WBI. This technique resulted in less radiotherapy fractions. Typically, SIB can be performed with conventional fractionation as well as hypofractionation. Thus, the total dose per fraction of SIB to the tumor bed could be approximately 2.4–2.6 Gy for conventional fractionation and 3.0–3.2 Gy for hypofractionation [11]. Many studies have revealed that both conventional fractionation and hypofractionation with SIB seem to be safe and feasible [12-25]. The toxicities and cosmetic outcomes of hypofractionation with SIB (H-SIB) were evaluated and were comparable to those of conventional fractionation with SIB (C-SIB) [26,27]. Recently, we reported the results of acute and late toxicities and cosmetic outcomes between the C-SIB and H-SIB groups in early-stage breast cancer patients at our institution. H‐SIB provided better cosmetic outcomes than C‐SIB [28].
Despite favorable toxicities and cosmetic results, the long-term oncologic outcomes of H-SIB are essential and still lacking. Therefore, we further analyzed and compared the oncologic outcomes between C-SIB and H-SIB in early-stage breast cancer patients who received BCS at our institution. The primary objective was to evaluate and compare local control (LC) between both groups. The secondary outcomes were locoregional control (LRC), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS).
Materials and Methods
We retrospectively collected data from early breast cancer patients who received adjuvant WBI with the SIB technique at King Chulalongkorn Memorial Hospital, from January 2008 to December 2017. The inclusion criteria were women ≥18 years old with histopathologically confirmed stage I–II (AJCC 7th edition) breast cancer who underwent adjuvant WBI with SIB after BCS. Exclusion criteria included evidence of skin invasion by the tumor, bilateral breast cancer, history of connective tissue disease, previous treatment with radiation therapy, postmastectomy, concomitant chemoradiation, history of other cancers, or loss to follow-up after completion of the radiotherapy session.
Radiotherapy was delivered with either C-SIB or H-SIB. Patients in the C-SIB group received WBI of 50 Gy in 25 daily fractions with an SIB of 0.6 Gy per fraction to the tumor bed, for a total dose of 65 Gy in 25 fractions. The corresponding dose in the H-SIB group was 43.2 Gy in 16 daily fractions with the same dose of SIB, resulting in a total dose of 52.8 Gy in 16 fractions.
All patients were treated with opposing tangential fields to the affected breast in the supine position using a breastboard (MT350; CIVCO Medical Solutions, Coralville, IA, USA). Conventional two-dimensional (2D) planning was performed between 2008 and 2011. Afterward, three-dimensional (3D) planning was delivered. A computed tomography (CT) simulation was performed, and data were transferred to Eclipse Planning System version 8.10 (Varian Medical system, Palo Alto, CA, USA). For WBI, photon 6 MV were used in all patients. Tumor bed boost volume was defined by surgical clips or postoperative seroma and correlated with preoperative imaging. A 2-cm margin around the tumor bed volume was irradiated with an en-face electron beam of 6–12 MeV. Regional nodal irradiation (RNI) was given to patients with node positivity, patients who did not have surgical node staging, and some patients with other high-risk features (e.g., clinical N0 but no surgical node staging or lymphovascular invasion). The anterior supraclavicular field typically covered supraclavicular, axillary node level II, III and some parts of axillary node level I.
This study protocol was approved by the ethics committee of Faculty of Medicine, Chulalongkorn University (No. 172/62).
1. Statistical analysis
To compare LC, LRC, DMFS, DFS and survival outcomes between C-SIB and H-SIB, we used Kaplan-Meier and log-rank tests. The base of follow-up time was the first date that the patient received irradiation, LC was defined as the time to ipsilateral breast tumor recurrence. LRC was defined as the time to any local or regional recurrence. DMFS was defined as time to distant failure. DFS was defined as the time to any disease progression or death. OS was defined as time to death from any cause. A p-value <0.05 was considered statistically significant. Descriptive analysis, the Mann-Whitney U test and Fisher exact test were used to report patient and tumor characteristics, adjuvant treatment and differences in these variables between the two groups. The prognostic impact for local recurrence and death of variables were assessed in univariate analysis and multivariate analysis by Cox proportional hazards modeling. Variables that tended to be significant in univariate analysis (p < 0.10) were selected for multivariate analysis. The propensity scores matching using covariate adjustment method—i.e., age, RNI, stage grouping, nodal (N) stage, chemotherapy status, and histologic grade—was used to reduce the effect of confounders on outcomes following H-SIB and C-SIB and used number of neighbors to calculate the matched outcomes. Stata version 15.1 (Stata Corp., College Station, Texas) and SPSS version 22 (IBM Corp., Armonk, NY, USA) were used for analysis.
Results
1. Patients, tumor characteristics and adjuvant treatment
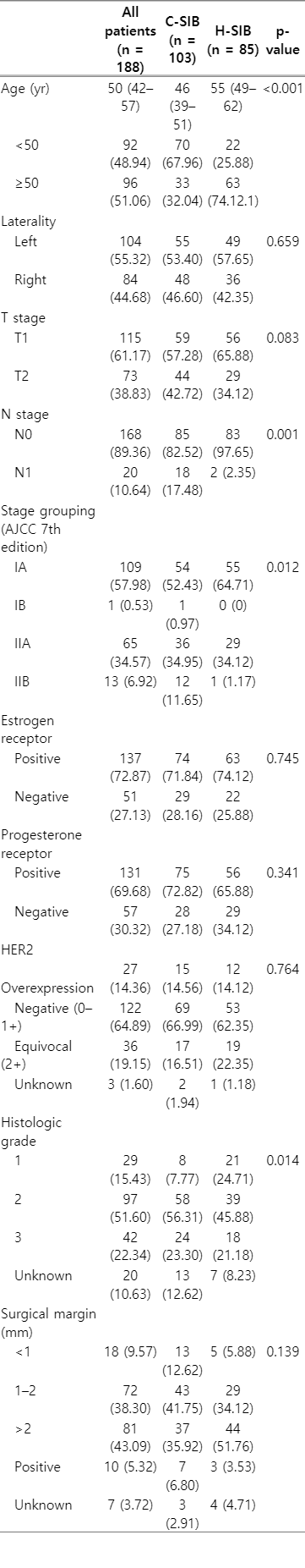
A total of 188 patients were included in this study: 103 patients were treated with C-SIB, while 85 patients received H-SIB. The median age of the entire cohort was 50. The majority of both groups were diagnosed with stage T1, and the most common histology subtype was invasive ductal carcinoma. There was a significantly higher proportion of patients who were ≥50 years old in the H-SIB group, while the C-SIB group had a significantly higher proportion of positive nodes, advanced stages and more high-grade tumors (Table 1).
Negative surgical margins were achieved in approximately 91% of patients, and 6.8% and 3.5% of patients had positive surgical margins in the C-SIB and H-SIB groups, respectively. For information of surgical node staging, 78 and 102 patients had axillary node dissection and sentinel node biopsy, respectively. Other eight patients did not have surgical node staging or the type of axillary node surgery was not specified. The majority of patients were estrogen receptor (ER) or progesterone receptor (PR) positive, and most of them received anti-hormonal treatment. Overall, HER2 overexpression was found in 14.4% of patients, and only 5.9% of patients received trastuzumab. Approximately one-fifth of patients had equivocal or unknown HER2 results with no further molecular test confirmation since trastuzumab could not be reimbursed at that period. The proportion of patients who received chemotherapy in the C-SIB group was significantly higher than that in the H-SIB group, 71.8% and 49.4%, respectively (Tables 1, 2). The most common chemotherapy regimen for both groups was anthracycline+cyclophosphamide. Other regimens included anthracycline+cyclophosphamide+taxol, taxol+cyclophosphamide, cyclophosphamide+methotrexate+fluorouracil, fluorouracil+anthracycline+cyclophosphamide, fluorouracil+epirubicin+cyclophosphamide, carboplatin+taxol, and taxol only.
Almost 90% of patients received the 3D radiation technique. Only 15 and 6 patients were treated with conventional techniques in the C-SIB and H-SIB groups, respectively. Only one patient in the C-SIB group was treated with intensity-modulated radiotherapy (IMRT), while none in the H-SIB group received IMRT. Corresponding with the proportion of node positivity, 21.4% of the C-SIB group received regional node irradiation, which was significantly higher than in the H-SIB group (only 3.5%) (Table 2). Treatment plan for patients who received regional node irradiation composed of two tangential fields and a supraclavicular field.
2. Oncologic outcomes
The median follow-up time was 87 months (range, 18 to 147 months; interquartile range [IQR], 65 to 112 months) for the entire cohort, 83 months (range, 18 to 147 months; IQR, 62 to 116 months) in the C-SIB group and 89 months (range, 36 to 132 months; IQR, 67 to 107 months) in the H-SIB group. Local recurrence occurred in four patients (3.9%) in the C-SIB group and two patients (2.6%) in the H-SIB group, with 7-year LCs of 95.8% and 98.6% in the C-SIB and H-SIB groups, respectively (p = 0.619) (Fig. 1A). Regional recurrence occurred in four patients (3.9%) in the C-SIB group and three patients (3.5%) in the H-SIB group. The 7-year LRC was 95.8% in the C-SIB group and 97.4% in the H-SIB group (p = 0.964) (Fig. 1B). Ten patients (9.7%) in the C-SIB group and three patients (3.5%) in the H-SIB group developed distant metastasis in which the common sites were the bone, lung and liver. Seven-year DMFS was 89.9% and 95.9% (p = 0.111), and 7-year DFS was 84.2% versus 95.4% (p = 0.176) in the C-SIB and H-SIB groups, respectively (Fig. 2A, 2B). Death from any cause occurred in eight patients (7.8%) in the C-SIB group and one patient (1.9%) in the H-SIB group, with 7-year OS rates of 92.1 % and 98.2% in the C-SIB and H-SIB groups, respectively (p = 0.040) (Fig. 2C).
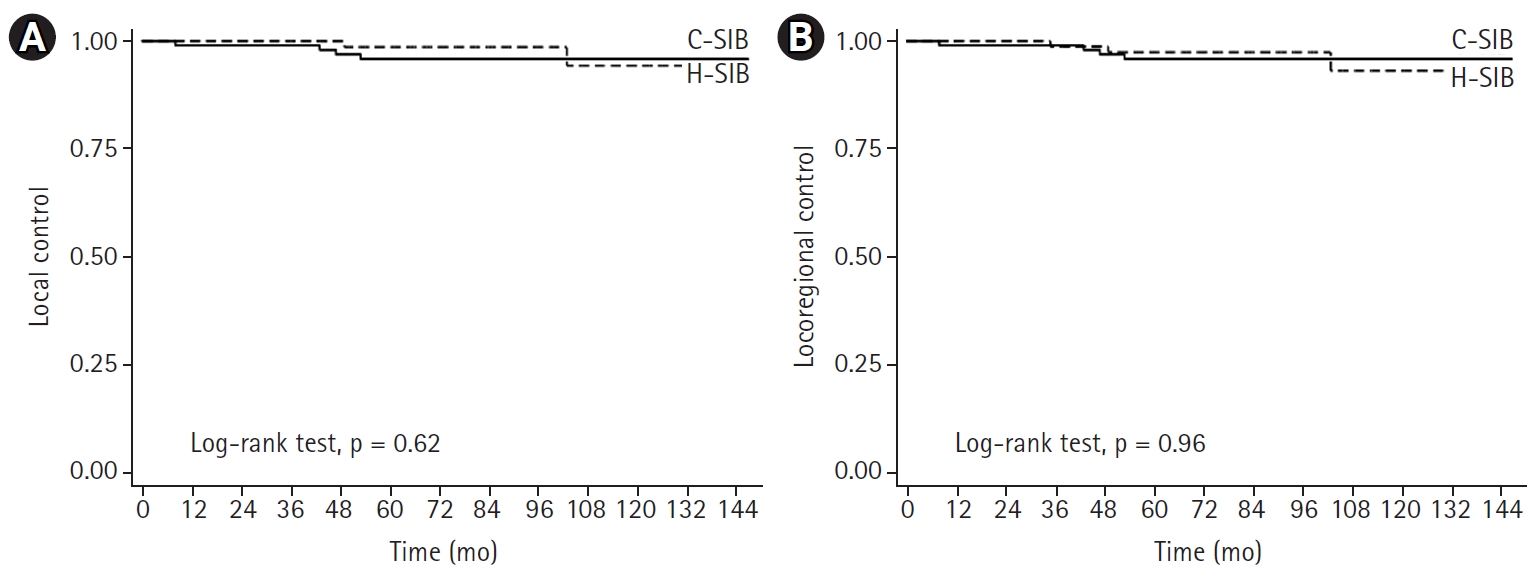
Tumor control outcomes of patients who received either C-SIB (solid line) or H-SIB (dashed line). (A) Local control. (B) Locoregional control. C-SIB, conventional fractionation with simultaneous integrated boost; H-SIB, hypofractionation with simultaneous integrated boost.
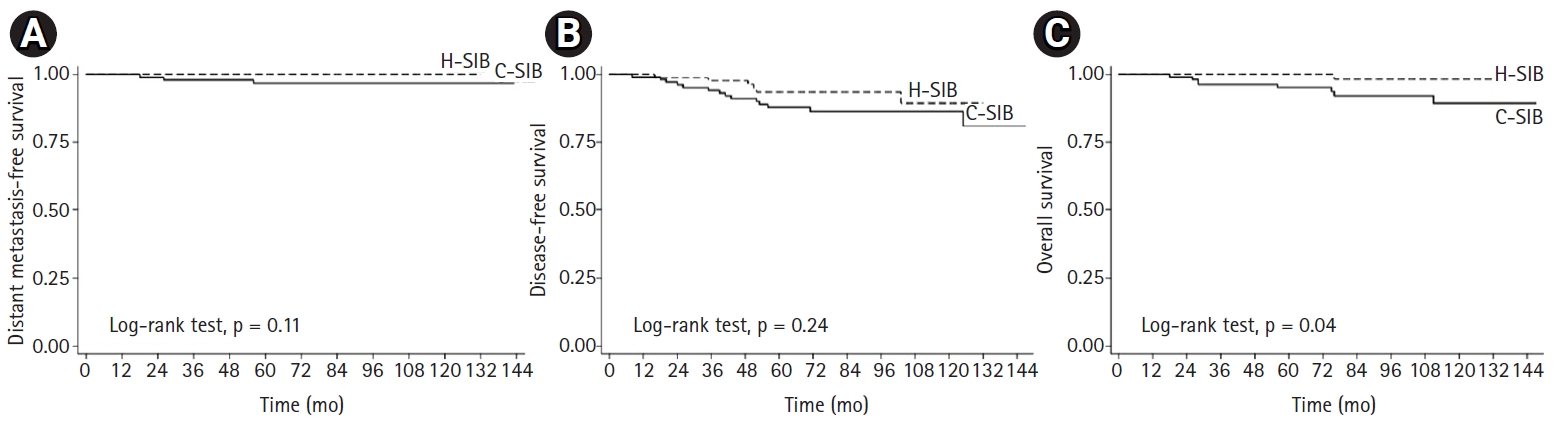
Survival outcomes of patients who received either C-SIB (solid line) or H-SIB (dashed line). (A) Distant metastasis-free survival. (B) Disease-free survival. (C) Overall survival. C-SIB, conventional fractionation with simultaneous integrated boost; H-SIB, hypofractionation with simultaneous integrated boost.
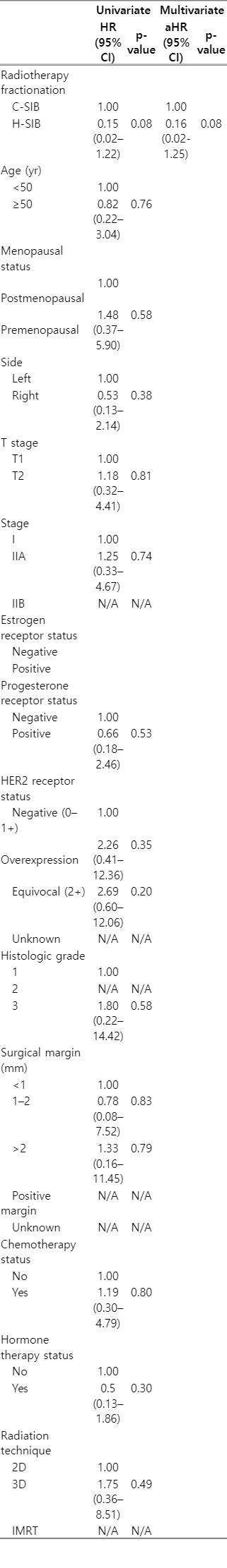
For the association between variables and local recurrence or death, due to small sample size, some factors could not be evaluated. HER2 receptor overexpression status and invasive lobular carcinoma showed a significant association with local recurrence, and the hazard ratios (HRs) were 15.4 and 14.2, respectively (p = 0.02 and p = 0.02), while estrogen receptor positivity and hormonal therapy tended to be associated with less local recurrence in univariate analysis. However, only invasive lobular carcinoma was found to be significantly associated with local recurrence in multivariate analysis (HR = 16.01, p = 0.03) (Table 3). For death, univariate analysis showed that H-SIB tended to be associated with better survival (HR = 0.15, p = 0.08). However, after adjusting for other factors, different SIB technique did not show a significant impact on survival (Table 4).
To reduce the bias of imbalanced factors between both groups, 82 out of 188 patients were included for propensity score matching. There was no significant difference in baseline patients and tumor characteristics and adjuvant treatment between both groups (Supplementary Tables S1, S2). The 7-year OS in C-SIB and H-SIB groups were 84.4% and 96.9%, respectively. There was no statistical difference in OS between both groups (HR = 0.16, 95% confidence interval 0.02–1.39, p = 0.097). Both 7-year LC and LRC in C-SIB and H-SIB groups were 94.9% and 100%, respectively. Due to no event of local recurrence in H-SIB group after performing propensity score matching, the difference of LC and LRC between both groups could not be analyzed (Supplementary Figs. S1, S2).
3. Cosmetic outcomes
As we reported in our previous publication, the cosmetic outcomes were satisfactory. Patient-rated cosmetic outcomes in both groups were mostly “excellent,” 40.3% and 45.6%, while “poor” outcomes were 5.3% and 1.8% in C-SIB and H-SIB group, respectively. In addition, more than half of corresponding satisfaction in both groups was “very satisfied” while “unsatisfied” was found only 0% and 1.8% in C-SIB and H-SIB group, respectively [28].
Discussion and Conclusion
This retrospective cohort study was performed to compare long-term oncologic outcomes between C-SIB and H-SIB in early-stage breast cancer. Various dose fractionations have been used in many published studies to evaluate the outcomes of H-SIB and C-SIB. The biological effective dose (BED) was calculated with alpha over beta: α/β = 4 Gy for tumor control [29,30] and α/β = 3 Gy for late normal tissue toxicity [31]. Our study applied equivalent doses in 2 Gy fractions (EQD2) of 64.24 Gy (α/β = 4 Gy) and 66.53 (α/β = 3 Gy) in the H-SIB group and 71.5 Gy (α/β = 4Gy) and 72.8 Gy (α/β = 3Gy) in C-SIB group. Despite high EQD2 to the tumor bed in both groups, our previous publication rarely found unfavorable toxicities or cosmetic outcomes [28]. In addition, regardless of the lower BED of the H-SIB group, our findings show comparable LC, LRC, DMFS and DFS between the two groups. Although significantly better OS was found in the H-SIB group owing to better prognostic factors (Table 1), there was no statistical difference in OS after propensity score matching. A significantly higher proportion of poor prognostic factors, i.e., younger age, greater node positivity, advanced group stage, and high-grade histology were found in the C-SIB group, which could directly affect OS. After adjusting for other prognostic factors, radiation therapy technique was not significant factor for OS. However, only invasive lobular carcinoma was significantly associated with local recurrence in multivariate analysis. Nonetheless, our findings were in line with other studies that reported excellent tumor control and survival outcomes in both H-SIB [14,19] and C-SIB [12] Moreover, our results are also similar to historical data of sequential tumor bed boost following both conventional and hypofractionated WBI [9,10].
Breast cancer is one of the most common diseases that require radiation. In particular, in patients who undergo BCS, WBI is given as an adjuvant treatment in most cases. Since conventional fractionation requires more than a month of treatment, it is not only inconvenient for patients to go forth and back to receive treatment but also consumes radiotherapy medical resources, which are still scarce in some regions. According to the radiobiological parameter of breast cancer, its α/β is typically estimated to be approximately 4, which is less than that of most other malignant tumors [29,30]. The relatively low α/β suggests that a short course of treatment with a high radiation dose per fraction may be beneficial. Several studies have supported this postulation and showed that hypofractionation is not inferior to the conventional fraction in both toxicity and efficacy [8-10,30]. Thus, hypofractionated radiotherapy has become the well-accepted standard treatment in early-stage breast cancer.
To enhance LC, tumor bed boosts were incorporated with WBI [6,32-34]. As a consequence, the treatment course has to be extended by approximately 1–2 weeks. According to the low α/β, SIB, with the advantages of both biological effects and shorter treatment courses, were introduced for tumor bed boost [12,17,20]. Later, H-SIB gained popularity. Since the dose per fraction at the tumor bed boost for H-SIB is quite high, toxicities and cosmetic outcomes are of concern. However, previous H-SIB studies showed promising oncologic outcomes and acceptable toxicities [13,14,16,35]. Consistent with our institution’s findings, we confirmed excellent oncologic outcomes and favorable toxicities and cosmetic outcomes in the H-SIB group [28]. Therefore, H-SIB, which reduces the radiotherapy treatment course of both WBI and tumor bed boost, could be a good and acceptable option. Randomized controlled studies comparing H-SIB and C-SIB are ongoing. However, to our knowledge, the results have not yet been reported [35,36].
In addition to the moderate hypofractionated radiotherapy that we generally used, ultrahypofractionated WBI has also been studied. Results from the United Kingdom showed that the ultrahypofraction regimen, 26 Gy in 5 fractions over 1 week, was noninferior to moderate hypofractionation, 40 Gy in 15 fractions over 3 weeks, in terms of LC and toxicities [37]. However, the 5-year results might be too short to validate their findings. Therefore, ultrahypofractionated WBI should be used with caution, and moderate hypofractionation should remain the standard adjuvant WBI in most patients [38]. Moreover, the tumor bed boost in this study was administered sequentially in 5–6 daily fractions, which doubled the length of the radiotherapy course in the ultrahypofractionation group. To date, no data on SIB in ultrahypofractionation have been reported.
Although this study has provided insight into SIB in WBI, there were several limitations. First, the number of patients might be underpowered to demonstrate the difference in oncologic outcomes and might be too small to report the association of some variables with local recurrence or death. Second, late relapse could possibly occur in early-stage breast cancer especially in hormone-positive breast cancer. Reports with longer follow-up times are needed. Third, since this was a retrospective study, the imbalance of patient characteristics between the two groups, especially nodal stage, possibly affected the oncologic outcomes. However, in multivariate analysis, there was no significant predictor for OS, but invasive lobular carcinoma was associated with local recurrence. Also, there was no significant difference in OS between both groups after performing propensity score matching. Further prospective randomized studies are warranted to explore these limitations. The strength of this study included it has a long median follow-up time and a use of homogeneous radiation technique in a tertiary care center.
In conclusion, our results showed that H-SIB after BCS in early-stage breast cancer provided comparable locoregional control to C-SIB. To shorten the overall radiotherapy course, H-SIB is a feasible option in patients who require WBI with a tumor bed boost after BCS.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization: CL, MP; Funding acquisition: CL; Investigation and methodology: CL, CN, MP; Project administration: CL, MP; Resources: CL, MP; Supervision: CL; Writing of the original draft: CL, MP, CN; Writing of the review and editing: CL, CN; Software: MP, CN; Validation: CL, CN; Formal analysis: CL, MP, CN; Data curation: CL, CN; Visualization: CL, CN.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2021.00927.
Baseline patients and tumor characteristics
Adjuvant treatment
Local control outcome of patients who received either C-SIB (solid line) or H-SIB (dashed line) after propensity score matching. C-SIB, conventional fractionation with simultaneous integrated boost; H-SIB, hypofractionation with simultaneous integrated boost.
Locoregional control outcome of patients who received either C-SIB (solid line) or H-SIB (dashed line) after propensity score matching. C-SIB, conventional fractionation with simultaneous integrated boost; H-SIB, hypofractionation with simultaneous integrated boost.