Radiotherapy, volume reduction, and short-term surgical outcomes in the treatment of large myxoid liposarcomas
Article information
Abstract
Purpose
While tumor volume reduction following radiation has been documented in myxoid liposarcomas, it is unclear whether large tumors experience similar volume reduction to smaller tumors.
Materials and Methods
MRI studies performed before and after completion of pre-operative radiation therapy (RT) were examined. Tumor sizes were noted and categorized as large versus small based on size >10 cm. Tumor volumes were calculated, and operative duration and major wound complications were recorded.
Results
The median largest tumor dimension was 12.4 cm before RT and 8.7 cm after RT. The median tumor volume was 298.9 cm3 before RT and 106.9 cm3 after RT. There was no significant difference in the mean percent tumor volume reduction between large tumors and small tumors (p = 0.11, 56.3% vs. 64.5%). Operative duration most strongly correlated to post-RT MRI volume (R2=0.674, p<0.001). Despite volume reduction, tumors that were large on presentation were more likely to experience major wound complications post-operatively.
Conclusion
Radiation appears to be as effective at reducing myxoid liposarcoma tumor volume in large and small tumors. However, large tumors on presentation appear more likely to experience wound complications despite tumor volume reduction. Future studies should investigate disease-related outcomes as a factor of volume reduction in myxoid liposarcoma.
Introduction
Myxoid liposarcoma (MLS) is the most common subtype of liposarcoma and represents approximately 5% of all adult soft tissue sarcomas (STS) [1,2]. Characteristics of MLS include a t(12:16) translocation and perhaps a more favorable prognosis compared to other liposarcomas despite an unusual predilection for extrapulmonary metastasis [3,4]. Intermediate- and high-grade STS, including MLS, are typically treated with combination surgery and radiotherapy (RT), which have been shown to improve local control when compared to surgery alone. While RT for STS can be performed pre- and post-operatively, pre-operative RT has the advantages of fewer late complications and the potential to improve resectability prior to surgery, leading to a shift toward neoadjuvant RT in the treatment of STS in recent years [5–7].
Several historical studies have suggested that MLS may be more likely to respond to RT than other subtypes of STS, with high rates of regression and even reports of complete clinical response after RT [8–12]. However, older series are difficult to interpret due to inclusion of different subtypes of liposarcoma, changes in diagnostic criteria, and variations in radiation techniques. More recent studies have demonstrated an objective response of MLS to neoadjuvant RT with substantial reductions in tumor volume and changes in tumor morphology after RT as well as higher rates of local control after neoadjuvant RT and surgical resection when compared to other STS subtypes [13–15].
While the effects of RT in MLS have been previously described, the response of MLS to RT specifically based on initial tumor size has not been robustly evaluated. In STS, tumor size impacts ease of resection, rate of post-operative wound complications, and prognosis [16]. The differential response to RT in MLS tumors of different sizes has the potential to inform individualized treatment protocols as well as impact pre-operative planning, but studies specifically examining this are lacking. Accordingly, the purpose of this study was to compare the response of large and small MLS tumors to a standardized protocol of neoadjuvant radiation therapy and to assess surgical complications in these patients.
Materials and Methods
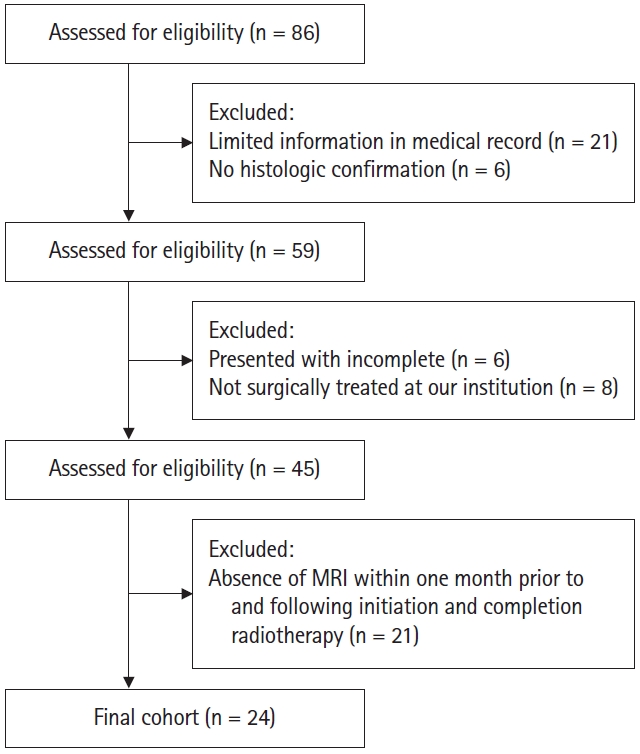
Following Institutional Review Board of Rush University Medical Center approval (ORA No. 21101407-IRB01), our institutional sarcoma database was retrospectively reviewed to identify all patients with MLS from 2000 to 2021. Initial query returned a total of 86 patients. Initial query returned a total of 86 patients. The following exclusion criteria were applied: those with limited information in the medical record (n = 21), those without histological confirmation of MLS (n = 6), those who presented with previous incomplete excisions from outside hospitals without tumor size information (n = 6), and those who were not primarily treated surgically for their MLS at our institution (n = 8). Patients were additionally excluded if magnetic resonance imaging (MRI) of their tumors were not available both within one month prior to initiation of radiotherapy (RT) and within one month after completion of RT (n = 21) (Fig. 1). After the above exclusion criteria were applied, the records of 24 patients were reviewed retrospectively.
Basic patient and tumor variables were collected, including age, sex, race, and primary tumor location. The presence of a round cell component on the histological specimen was noted and recorded as a categorical variable. In two cases, information regarding presence of round cell component was unavailable and treated as missing data. Race was coded as a categorical variable defined as Caucasian and non-Caucasian. The majority of tumors were located in the thigh (91.7%, n = 22) and the remaining two (8.3%) were located in the calf. Operative duration was recorded in minutes and major wound complications were defined as described by O’Sullivan et al [17]. These complications were defined as a secondary operation under anesthesia for wound repair or non-operative wound management involving invasive procedures, readmission for antibiotics or wound care, or persistent deep packing for 120 days. Operative duration was unavailable in one patient.
Tumor size measurements in craniocaudal, transverse, and anteroposterior dimensions were recorded from available radiologists’ reports. When a measurement was not noted in the report, one author (LL) recorded the missing size measurements. All pre-RT tumor sizes were recorded from MRI studies performed within one month prior to the initiation of RT. All post-RT tumor sizes were recorded from MRI studies performed within one month after completion of RT. Tumor volume was calculated using the ellipsoid formula:
volume = 4/3 × π × a × b × c,
where a, b, and c are the tumor dimensions in craniocaudal, transverse, and anteroposterior dimensions. True tumor specimen size was recorded as the largest dimension on pathology after surgical resection. Percent tumor necrosis was recorded from corresponding pathology reports upon final surgical resection. All pathology specimens were reviewed by board-certified, fellowship-trained musculoskeletal pathologists with expertise in sarcoma. Tumor necrosis was estimated by the pathologists according to a departmental protocol which relies on the gross appearance and percentage of necrotic cells in various sections. In these irradiated tumors, necrosis percentage is used as an indicator of response to treatment; therefore, both necrosis and fibrosis are included. Tumor necrosis data was available in 22 of 24 patients.
Large tumors were determined to be those with a largest dimension greater than 10 cm and small tumors were determined to be those with a largest dimension smaller than 10 cm. Tumors were categorized as large and small according to pre-RT MRI largest dimension, post-RT MRI largest dimension, and final tumor specimen size. While a tumor size cutoff of 5 cm is more commonly used for staging purposes, a tumor size of 5 cm typically does not impose significant operative challenges. Therefore, we focused on tumors greater than 10 cm in size as these cases may experience greater benefit from volume reduction for surgical complexity.
All patients underwent wide, local excision of their MLS within 6 weeks of completion of neoadjuvant RT and all margins were negative. All patients underwent neoadjuvant RT prior to surgery, receiving a cumulative 50 Gy over 25 fractions. Four patients underwent interdigitated chemotherapy and radiation. The chemotherapy regimen was a combination of doxorubicin and ifosfamide in all four patients.
Categorical variables were described using frequencies and percentages and compared with chi-squared or Fisher exact test. Continuous data were reported as mean with range or median with interquartile range (IQR) and compared using Mann-Whitney U Test or paired t-test. Linear regression analysis was performed to analyze the relationship between volume reduction and tumor necrosis as well as between various size and volume measurements with operative duration. Statistical significance was set to p < 0.05, and all analyses were performed on SPSS version 26.0 (IBM, Armonk, NY, USA) and RStudio version 1.4 (Integrated Development for R. RStudio; PBC, Boston, MA, USA).
Results
The median largest tumor dimension on pre-RT MRI was 12.4 cm (IQR 7, 16) and the median tumor volume prior to radiation for the entire cohort was 298.9 cm3 (IQR 71.4, 830.8). Both MLS size and volume were significantly reduced following preoperative RT in the entire group (both p < 0.001). After RT, the median largest tumor dimension on MRI was 8.7 cm (IQR 6.3, 14) and the median tumor volume was 106.9 cm3 (IQR 30.0, 362.6) (Fig. 2). Tumors with largest dimension greater than 10 cm on pre-RT MRI had a mean volume of 829.8 cm3 (range, 220.2 to 2,813.4) whereas the smaller tumor group had a mean volume of 76.2 cm3 (range 4.0 to 241.9 cm3) (p < 0.001). Patient age (p = 0.68), gender (p = 0.81), race (p = 0.09), and presence of round cell component (p = 0.47) did not differ between the two groups. Pooled patient demographics and tumor characteristics can be seen in Table 1.

Axial magnetic resonance imaging (MRI) of myxoid liposarcoma with (A) largest dimension of 16 cm on presentation and (B) largest dimension of 14 cm after radiotherapy (RT). Axial MRI of myxoid liposarcoma with (C) largest dimension of 5.5 cm on presentation and (D) largest dimension of 2.1 cm after RT.
The mean absolute volume reduction was 409.0 cm3 (range, 112.5 to 1,100.2 cm3) in the large tumor group and 47.4 cm3 (range, -10.1 to 146.3 cm3) in the small tumor group. One tumor grew over the course of RT. There was no significant difference in the mean percent tumor volume reduction between the large tumor group and the small tumor group (p = 0.11). The mean percent tumor volume reduction in the large tumor group was 56.3% (range, 22.4% to 90.9%) compared to 64.5% (range, -18.4% to 91.7%) in the small tumor group. Relative percent volume reduction did not correlate with percent tumor necrosis on final specimen evaluation (R2 = 0.07, p = 0.23). There was also no difference in tumor necrosis seen in large tumors versus small tumors as determined by pre-RT MRI (p = 0.81).
Operative duration did not differ when tumors were categorized as large and small based on pre-RT MRI largest dimension (p = 0.07); however, operative duration was significantly greater when tumors were categorized based on post-RT MRI largest dimension (p = 0.006) as well as when categorized based on pathology tumor specimen size (p = 0.003) (Table 2). Linear regression analysis revealed significant correlation between operative duration and pre-RT MRI tumor volume (R2 = 0.606, p < 0.001), operative duration and post-RT MRI volume (R2 = 0.674, p < 0.001), and operative duration and tumor specimen size (R2 = 0.597, p < 0.001).
Only one patient required non-primary wound closure and underwent a flap reconstruction with a split thickness skin graft. There were six total major wound complications in the entire group. When tumors were categorized based on pre-RT MRI largest dimension, five of the six complications occurred in the large tumor group. When categorized based on post-RT MRI largest dimension, two of the six complications occurred in the large tumor group. And when categorized based on tumor specimen size, three of the six complications occurred in the large tumor group (Table 3). Low overall numbers precluded a powered statistical analysis. Individual patient and tumor characteristics can be seen in Table 4.
Discussion and Conclusion
The sensitivity of MLS to radiation therapy was first documented more than 50 years ago, and more recent studies have provided objective evidence of MLS tumor response to RT [10,11]. In our series, there was a significant reduction in tumor size and volume after neoadjuvant RT, with a proportional reduction in median tumor volume of 64%. This finding is consistent with previous studies that examined MLS tumor size reduction after RT. Pitson et al. [13] observed a 59% reduction in tumor volume in MLS after RT and other studies have consistently demonstrated large decreases in tumor volume after RT, highlighting the radiosensitivity of MLS [2,13,14,18,19].
The principle aim of this study was to examine the response to neoadjuvant RT in MLS tumors of different sizes. In soft tissue sarcoma, tumor size is an important prognostic factor and is associated with rates of local recurrence, post-operative wound complications, and overall survival [20–22]. It is important for clinicians to understand the effects of neoadjuvant RT in large versus small MLS tumors, as this has the potential to impact individualized treatment planning. To our knowledge, this is the first report to specifically examine the response to neoadjuvant RT in differently sized MLS tumors. In our study, there was no significant difference in mean percent volume reduction after RT between large and small MLS tumors. These results indicate that neoadjuvant RT is as effective at reducing the volume of large MLS tumors as small tumors. Large tumor size is associated with inferior disease-free survival and local control, and substantially decreased tumor size after RT likely contributes to the excellent local control rates observed in MLS [15,23–25]. Reducing the volume of large tumors also has the potential to facilitate surgical resection and reduce surgical morbidity, thereby improving functional outcomes without sacrificing local control. In this study, all resected tumor specimens had negative margins, even in very large tumors with greatest dimension greater than 20 cm at presentation. This indicates a benefit in terms of ease of resection after neoadjuvant RT which is important for treatment planning, especially for very large tumors or those in critical locations which may otherwise not be as amenable to resection. In addition to decreased tumor volume, changes in MLS tumor histology with more mature lipoma-like areas observed after RT and increased distance of the tumor from the neurovascular bundle after RT, may also contribute to an increase in resectability [14,18]. Furthermore, tumors that are initially deemed not resectable due to large size or proximity to the neurovascular bundle may become resectable after RT due to reduction in tumor volume, though further studies are necessary to determine if these results are applicable in primary unresectable MLS.
Our results also demonstrated no difference in tumor necrosis between large and small tumors after RT. This indicates that, in addition to a similar reduction in tumor volume after RT, the pathological response to RT is similar between large and small MLS tumors. Studies have suggested that rates of tumor necrosis correlate with clinical outcome, with a recent meta-analysis showing that rates of necrosis less than 90% are associated with increased recurrence risk and inferior overall survival in STS patients [26]. Based on the results of our study, clinicians can expect rates of necrosis in large MLS tumors that are comparable to small tumors after RT, and this may positively impact outcomes in these patients.
In this study, we observed significantly shorter operative times in the small tumor group when compared to the large tumor group. Smaller pre-RT size, post-RT size, and pathology specimen size were all correlated with shorter operative duration. However, the strongest correlation was with post-RT size, indicating that tumor size immediately prior to surgery is a more important factor than tumor size at presentation. Shrinking MLS tumors with neoadjuvant RT may contribute to ease of resection and lead to shorter operative times. Operative duration is an independent risk factor for surgical complications. A contemporary meta-analysis showed that the likelihood of developing a complication approximately doubled with operative times exceeding 2 hours and that there was a 14% increase in likelihood of complications for every 30 minutes of additional operative time [27]. Reducing tumor volume with neoadjuvant RT has the potential to reduce operative time and, in turn, reduce complications and costs associated with increased operative duration [28].
Despite advances in STS treatment, post-operative wound complications remain a major source of morbidity and have been reported in up to 56% of surgical cases [21]. There were six total major wound complications in our study with a rate of 25%, similar to prior reports. Previous studies have investigated factors that influence the risk for post-operative wound complications, including tumor size and location, timing of radiation pre- or post-operative, and radiation field size [16,21,29]. Tumor size and tumor volume have been found in multiple studies to be predictors of post-operative wound complications, and a study by Ziegele et al. [21] demonstrated that tumor volume may be a stronger predictor of complication risk than tumor size based on largest diameter. Larger volume of resection contributes to development of wound complications by leading to larger soft tissue dead space with increased potential for formation of seromas, hematomas, and infection. The significant reduction in both tumor volume and tumor size seen in our cohort after neoadjuvant RT has the potential to reduce post-operative wound complications in these patients. It is important to note that in our study, most wound complications occurred in the large tumor group when this was assessed based on pre-RT size. However, when evaluating complications based on pathology specimen size after resection, the number of complications in the large and small tumor groups were similar, though small numbers prevented statistical analysis. These results indicate that tumors that are large on presentation may still be more likely to have wound complications after resection, regardless of reduction in tumor size after RT. This is likely due to the larger radiation field size needed for neoadjuvant RT in larger tumors. The association between neoadjuvant RT and increased wound complications has been well documented, and the rate of complications increases with the magnitude of the radiated area [16,21,29]. It is important for clinicians to recognize that even if RT leads to reduced tumor size, tumors that are large prior to RT may still be more prone to post-operative wound complications.
This study does have several limitations, principally, the retrospective nature of the study and the small sample size. STS are rare tumors and our sample size was further restricted by limiting our population to patients with MLS who had undergone neoadjuvant RT, though we note that our cohort is among the largest in similar studies. There is also potential error in MRI volume measurements, though all tumor volume measurements were reported by board-certified radiologists specially trained in musculoskeletal pathology or performed by a single researcher to reduce interobserver variability. The use of neoadjuvant chemotherapy in some patients may also affect the ability to draw conclusions from histological evaluation of tumor response, as chemotherapy itself causes tumor necrosis. However, the identical protocol for neoadjuvant RT in all patients is a strength of the study. Finally, we did not examine long-term outcomes including disease-free and overall survival as this was outside the scope of this study. Further investigation with longer follow-up is necessary to determine the effect of RT on long-term outcomes in large versus small MLS tumors.
Our results demonstrate a significant reduction in tumor volume and size seen after RT in MLS and support the use of neoadjuvant radiation therapy in these tumors. Based on our data, neoadjuvant RT is as effective at reducing tumor volume and generating tumor necrosis in large tumors as in small tumors. Use of RT in large MLS tumors has the potential to increase ease of surgical resection as well as reduce operative time; however, large tumors on presentation seem more prone to major wound complications regardless of volume and size reduction. Additional studies are required to further examine the effect of neoadjuvant RT on rates of complications and on oncologic outcomes in large MLS tumors so that clinicians may be as informed as possible when creating individualized treatment protocols for these patients.
Notes
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board (ORA No. 21101407-IRB01).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.