 |
 |
AbstractPurposeConventionally fractionated radiotherapy (CRT) is widely applied for the treatment of high-risk prostate cancer. Pelvic node irradiation improves control of the disease. Although the therapeutic guidelines support the use of hypofractionated and accelerated radiotherapy (HypoAR), this is addressed to prostate and seminal vesicles. At the same time, the safety and efficacy of HypoAR for pelvic node irradiation remain obscure.
Material and MethodsIn a phase II study, we evaluated the feasibility of pelvic HypoAR in 22 high-risk prostate cancer patients. The RT scheme delivers 14 consecutive fractions of 3.67 Gy (total 51.38 Gy) to the prostate, 3.5 Gy (total 49 Gy) to the seminal vesicles, and 2.7 Gy (total 37.8 Gy) to the lymph nodes, using image-guided volumetric modulated arc therapy. A comparative radiobiological analysis of dose-volume histogram is performed (HypoAR vs. hypothetical equivalent CRT regimens, without and with time correction).
ResultsOur clinical experience shows impressively low early and short-term late toxicities, without any grade III events, within a median follow-up of 30 months. Only one biochemical relapse was recorded 30 months after irradiation. In radiobiological analysis, considering an ╬▒/╬▓-value of 4 Gy and a ╬╗-value of 0.2 Gy/day for late effects, all comparisons predicted significantly lower toxicity for the HypoAR regimen (p < 0.05). For early toxicities (╬▒/╬▓ = 10 Gy), a ╬╗-value lower than 0.4 Gy/day favors the HypoAR regimen, which is along with the clinical results.
IntroductionHigh-risk prostate cancer is an aggressive disease with an increased probability of relapse after treatment [1]. According to the National Comprehensive Cancer Network (NCCN), high-risk patients are defined as those with extra-prostatic invasion and/or prostate specific antigen (PSA) >20 ng/mL and/or Gleason score higher than 7 [2]. Radiotherapy is the primary treatment modality for this subgroup of prostate cancer patients. Although the probability of death at ten years after high radiation dose schedules is below 10%, the relapse rate is far higher [3]. The risk of lymph node involvement in this high-risk group of patients is quite high, exceeding 60% when all the above three conditions are met [4], so that pelvic irradiation is a useful therapeutic option in patients treated with radical radiotherapy. In a recent randomized trial, pelvic irradiation improved disease-free and distant metastasis-free survival [5]. Moreover, the quality of life of patients receiving pelvic irradiation is not compromised, further supporting the choice of pelvic irradiation in patients with high-risk prostate cancer [6].
The administration of radiotherapy fractions larger than 2 Gy (hypofractionation), may enhance the efficacy of radiotherapy in tumors with low radiobiological ╬▒/╬▓-ratio. Moreover, it allows important reduction of the treatment days for the benefit of patients, reducing at the same time the workload and waiting lists of overloaded radiotherapy departments. As radiobiological analysis of clinical data suggests that the ╬▒/╬▓-ratio of prostate cancer is between 1ŌĆō3 Gy, thus lower than the one of late responding tissues, prostate cancer emerges as an excellent candidate for hypofractionated radiotherapy [7]. Based on extensive clinical data accumulated from randomized trials, the ASTRO/ASCO/AUA radiotherapy guidelines for prostate cancer support the choice of moderately hypofractionated and accelerated radiotherapy regimens (HypoAR), with doses per fraction between 2.4ŌĆō3.4 Gy, while ultra-hypofractionated and accelerated regimens with fraction size Ōēź5 Gy are also suggested as an alternative [8]. When, however, pelvic radiotherapy is to be applied for high-risk prostate cancer patients, the evidence available to support HypoAR for pelvic nodes is limited. Proposed schemes use conventional radiotherapy for pelvic nodes and deliver a simultaneous integrated boost to prostate and seminal vesicles during the treatment time demanded to complete conventional pelvic irradiation [9,10].
The current study aims to provide preliminary clinical experience, and radiobiological analysis of a HypoAR scheme addressed to prostate, seminal vesicles, and pelvic lymph nodes. The potential hazard to organs-at-risk (OARs) from the regimen is compared to a hypothetical conventionally (2 Gy/fraction) radiotherapy (CRT) scheme that delivers the same biological equivalent dose to the tumor. The analysis is performed with and without time correction.
Materials and MethodsWe report preliminary clinical data, and radiobiological analysis of the first 22 patients enrolled in a single-arm prospective study of pelvic HypoAR for high-risk prostate cancer patients treated with radical pelvic radiotherapy. Nineteen out of 22 patients also received hormonal therapy with Luteinizing hormone-releasing hormone (LH-RH) agonists and bicalutamide for 12 months, starting 3 months before the onset of radiotherapy. The Institute Ethics and Research Committee of the University Hospital of Alexandroupolis has approved the study (No. ES10 24-10-2018). The patients gave written informed consent to participate in the trial and to anonymously use their clinical and laboratory data for scientific research and publication.
The primary objective of the study was to demonstrate that, in radiobiological analysis, pelvic HypoRT does not significantly increase early and short-term late gastrointestinal and genitourinary radiation-induced toxicities, compared to a CRT scheme applied on the same target and OAR volumes, using a volumetric modulated arc therapy (VMAT) technique. Secondary objectives were to study the early toxicities and short-term late toxicities (within a minimum follow-up of 18 months) at the clinical level.
The study recruited prostate cancer patients referred for radical radiotherapy. Patients should have histologically confirmed adenocarcinoma of the prostate, with performance status (PS) of 0ŌĆō1, and at least one of the following features: Gleason score Ōēź8, extracapsular invasion in prostate magnetic resonance imaging (MRI), PSA plasma levels above 20 ng/mL, node involvement in pelvic MRI. Exclusion criteria comprised, metastatic disease, previous radiotherapy in the pelvic region, previous chemotherapy or immunotherapy for any neoplasia, previous prostate or bladder surgery for benign diseases, history of inflammatory bowel disease, history of bladder or colorectal cancer, major heart, kidney, liver, autoimmune or psychiatric disorders, and PS Ōēź2.
Table 1 shows the patient and disease characteristics. Six patients (27.2%) had extra-prostatic extension at multi-parametric prostate MRI, one of them with detectable enlarged pelvic nodes (regional recurrence high risk). Nine patients (40.9%) had a Gleason score 8ŌĆō10. Thirteen patients (59%) had initial PSA levels above 20 ng/mL. The age of patients ranged from 62 to 80 years (median, 72.5).
The follow-up of patients is based on the monitoring of PSA every 6 months. Additional radiological tests are performed when symptomatology demands further evaluation, or at confirmation of biochemical relapse. The follow-up ranges from 18 to 36 months (median, 30). Acute radiation toxicity was scored using the National Cancer Institute (NCI) Common Toxicity Criteria Version 4.0 scale, while the LENT-SOMA toxicity scale was applied to score late radiation sequel.
1. ContouringPre-treatment instructions for an empty rectum and a comfortable full bladder were given to patients for computed tomography (CT) simulation and before each treatment session. The patients were scanned in a supine position in a CT-simulator, and a knee-fix device was used for immobilization. The images were transferred to Monaco treatment planning system (TPS) version 5.11.03 (Elekta CMS, Maryland Heights, MO, USA) to contour the structures of interest and the production of the treatment plans.
The delineation of the targets and OARs was made by the head of the department (MIK) and the production of plans by the radiotherapy physicist signing the report (CN), in order to minimize the inter-physician and inter-physicist variability. The prostate gland, seminal vesicles, and pelvic lymph nodes constitute the three distinct clinical target volumes (CTVs), while the bladder, rectum, and sigmoid are the OARs. A non-uniform margin was applied to the CTVs in order to create the planning target volumes (PTVs). Based on our experience with image-guided radiation therapy (IGRT), these margins were placed to 0.7 cm laterally, 0.5 cm anteriorly, and 0.3 cm posteriorly for prostate and seminal vesicles and corrected thereafter by the physician where necessary. Pelvic irradiation included the internal and common iliac nodes up to the upper margin of the fifth lumbar vertebra. The CTV margins to create the PTV were set at 0.5 cm laterally, anteriorly, and posteriorly.
2. Radiotherapy techniquePatients were treated with a 6-MV Elekta Infinity Linear Accelerator (Elekta, Stockholm, Sweden) endowed with an Agility head (Elekta), and the technique was VMAT with IGRT. The inversed plans were produced with Monaco TPS version 5.11.03 (Elekta CMS), and each PTV received at least 95% of the prescribed dose to 98% of its volume. Before each radiation treatment, a cone-beam computed tomography (CBCT) was carried out by Elekta platform Synergy kV CBCT (XVI) to check and adjust the position of patients. All patients received 51.38 Gy to the prostate with 3.67 Gy per fraction, 49 Gy to the seminal vesicles with 3.5 Gy per fraction, and 37.8 Gy to the lymph nodes with 2.7 Gy per fraction, for a total of 14 fractions, five fractions per week, within 18 days. Fig. 1 shows the dose distributions of each irradiated PTV for each prescribed dose.
In contrast to the HypoAR regimen that treats each target simultaneously with distinct fractionation, the hypothetical CRT scheme delivers all therapy in 2 Gy fractions to all three targets. To give the same equivalent dose in 2 Gy (EQD2) as the HypoAR, the CRT regimen must be evolved in three phases with distinct treatment plans. The first phase includes the prostate, seminal vesicles and lymph nodes, the second the prostate and seminal vesicles, and the third the prostate only.
3. Radiobiological considerations ŌĆō normalized biological dose-volume histograms (DVHs)The formulas of normalized total dose without and with time correction (NTD and NTD_T) [11,12], also known as EQD2, are:
where D is the total physical dose, d is the dose per fraction, ╬▒/╬▓ is the ratio that provides the dose in Gray where cell killing from linear and quadratic components of the linear-quadratic equation are equal, ╬╗ is the estimated daily dose consumed to compensate for rapid tumor repopulation, Tc is the number of days required for the delivery of the NTD using conventional fractionation and To is the number of days required for the delivery of the accelerated scheme.
These equations were applied to the HypoAR prescription dose for each PTV in order to calculate the NTD and NTD_T. For prostate cancer, an ╬▒/╬▓-value of 2 Gy was applied for analysis [13,14]. The NTD calculated for prostate, seminal vesicles, and lymph nodes was 74 Gy, 68 Gy, and 44 Gy, respectively. For a hypothetical CRT scheme, the delivery of these total doses demands 51, 46, and 30 days, respectively. As the overall treatment time (To) for HypoAR scheme was 18 days for all three targets, the acceleration applied by the HypoAR scheme was 33, 28, and 12 days for prostate, seminal vesicles, and lymph nodes, respectively. The ╬╗-value was assumed to be 0.2 Gy/day because prostate cancer is a slowly growing tumor, although higher values may apply. Considering a ╬╗-value of 0.2 Gy/day, the NTD_T for cancer cells growing in the prostate, seminal vesicles, and lymph nodes areas was 80 Gy, 74 Gy, and 46 Gy, respectively.
For the radiobiological analysis of early toxicities from bladder mucosa, rectum, and sigmoid early toxicities, an ╬▒/╬▓ ratio of 10 Gy was applied, while for late toxicities, we used a value of 4 Gy [15ŌĆō17]. Regarding the effect of treatment acceleration on late responding tissues, a ╬╗-value of 0.2 Gy/day was considered [18]. For early mucosa toxicities, the ╬╗-value is obscure, so we assumed a range for ╬╗-value between 0.2-0.8 Gy/day [19,20]. Therefore, the NTD and NTD_T for late responding tissues included in the PTV (100% of dose) was 65 Gy and 72 Gy, respectively, while for early responding tissues, this was 58.5 Gy and 65ŌĆō85 Gy considering the range of ╬╗-value.
4. Conventional planningCRT plans were produced with prescription doses that would deliver the same NTD or NTD_T to the targets of interest. CRT planning was performed on the same contouring of targets and OARs used for the actual HypoAR plans. As was mentioned above, the CRT schedule contained a three-phase regimen. For the NTD-planning, an initial VMAT arc, in the first phase, included prostate, seminal vesicles, and lymph nodes for 44 Gy (2 Gy/fraction). In the second phase, a boost arc for an extra 24 Gy to the prostate and seminal vesicles (sum dose 68 Gy). Finally, in the third phase, a boost arc is confined to PTV prostate for an extra 6 Gy, 2 Gy/fraction (sum dose 74 Gy). Similarly, for NTD_T-planning, the first phase administered 46 Gy, the second for 28 Gy (sum dose 74 Gy), and the third for 6 Gy (sum dose 80 Gy). Each cost function that was used in delivered plans was exactly used in the three-phase plans, customized for each prescription dose.
5. Data processingTo compare dose distributions for the different fractionations, the raw data from each point of cumulative physical DVHs (pDVHs) (for PTVs and OARs) were extracted in an excel worksheet. In this data set, the NTD and NTD_T equations were applied to create complete biological DVHs (bDVH) of HypoAR and CRT schemes.
6. Statistical analysisThe primary endpoints of this phase II study was to prove that VMAT-HypoRT is not inferior in terms or early and late toxicities in radiobiological analysis, compared to VMAT-CRT. Given the favorable results obtained in a previously performed pilot study (unpublished data), showing that grade 3 rectal toxicity was lower than 10%. The SimonŌĆÖs two stage method was used to calculate the sample size based on rectal toxicity estimates. We considered 5% as the acceptable levels for rectal toxicity Ōēź3. Unacceptable toxicity with 80% power and 5% significance level was set at 20%. The model suggested a sample size of 20 patients, which is also considered reasonable taking also into account the predicted rate of recruitment of patients and the minimum follow-up of 18 months demanded [21].
The statistical analysis was performed with the PRISM 8 (GraphPad Software Inc., San Diego, CA, USA). More specifically, the paired two-tailed t-test was applied for the comparison of the two regimens. Four dose-points of the bDVH chosen for the comparison: D50%, D35%, D25%, and D15%, where Dx% is dose delivered to the x% of the organ volume [22]. A p-value <0.05 was considered for significance.
Results1. Clinical experienceEarly toxicities were impressively low. Dysuria grade 1 was noted in 6/22 and grade 2 in 1/22 patients. Urinary frequency grade 1 was noted in 6/22 patients. Four patients out of 22 developed grade 1, and 3/22 grade 2 proctitis. Diarrhea grade 1 was reported by 7/22 and grade 2 by 5/22 patients, which was settled within a week from the end of therapy. Within a median follow-up of 30 months, late toxicities are confined to 1/22 cases with grade 1 dysuria and 3/22 with grade 1 frequency. No toxicities related to the rectum, sigmoid, or bladder have been reported.
One patient died from intercurrent disease. All other patients are alive with no evidence of local or metastatic disease. One biochemical relapse occurred 30 months after irradiation.
2. Early toxicity radiobiological analysis
Tables 2 and 3 show the mean NTD and NTD_T, the standard deviation, the range, and the p-value for all OARs for early effects, using a range of ╬╗-values between 0.2ŌĆō0.8 Gy/day. Considering the NTD and NTD_T for a ╬╗-value of 0.2 Gy/day (Table 2), all data from bladder, rectum and sigmoid, favor the HypoAR regimen over the CRT and the differences are significant (p < 0.05). When, however, the upper ╬╗-value of 0.8 Gy/day is considered (Table 3), early responding mucosa of all three organs receives an NTD_T that is higher when the HypoAR regimen is applied. Fig. 2 shows graphically the NTD and NTD_T DVHs for ╬╗ values of 0.2 Gy/day and 0.8 Gy.
Taking into account the fact that the clinically observed early toxicities from the bladder, rectum, and sigmoid were very low in the HypoAR regimen, thus at worst equal to the one expected from CRT, we calculated the maximum ╬╗-value for early responding tissues that would give an NTD_T DVH curve proximal to the CRT curve.
Using linear interpolation, we estimated the ╬╗-value that would give an NTD_T DVH HypoAR curve close to the DVH curve of the CRT. Focusing on the 50% organ volume NTD_T, we estimate the ╬╗-values are 0.31 Gy/day, 0.47 Gy/day, and 0.32 Gy/day for bladder, rectum, and sigmoid, respectively. Focusing on the 15% organ volume NTD_T, the ╬╗-values are 0.61 Gy/day, 0.74 Gy/day, and 0.38 Gy/day for bladder, rectum, and sigmoid, respectively. Taking into account the low early toxicity observed in the clinical practice, we postulated that the ╬╗-value of pelvic early responding tissues is lower than 0.4 Gy/day.
2. Late toxicity radiobiological analysis
Table 4 shows the comparison of NTD and NTD_T for late effects of all OARs for ╬╗ = 0.2 Gy/day. For bladder and sigmoid, HypoAR NTD_T50 and CRT NTD_T50, there is no significant difference. However, all the other comparisons are strikingly lower in the HypoAR regimen (p < 0.05). Fig. 3 shows the NTD and NTD_T DHVs graphically for ╬╗ values of 0.2 Gy/day.
Discussion and ConclusionExternal beam HypoAR with various fractionation schemes ranging from 2.5 to 6.1 Gy per fraction are well-established regimens for localized irradiation of the prostate and/or seminal vesicles. Prophylactic pelvic node irradiation is, however, recommended for high-risk patients, as this improves the biochemical-free and disease-free survival [5]. Even after pelvic lymphadenectomy, irradiation of pelvic nodes in histologically confirmed node-positive disease improves the survival of patients [23]. Pelvic radiotherapy is always indicated for patients with radiologically detectable nodal disease scheduled for radical radiotherapy. Intensity-modulated radiation therapy (IMRT) techniques allow the administration of high radiation doses up to 56 Gy EQD2 with a modest side effect profile [24].
The experience reported on HypoAR regimes for high-risk prostate cancer demanding pelvic irradiation is limited. Several studies attempt hypofractionated schemes to the prostate gland and conventional fractionation to lymph nodes. The outcomes of these reports were acceptable despite the small samples and the short follow-up time [9,10,25-27]. A recent study reports 105 patients treated with combined androgen deprivation therapy (ADT) and pelvic HypoAR [28]. The prescribed dose to prostate PTV was 60 Gy with 3 Gy/fraction, and for PTV lymph nodes were 44 Gy, 2.2 Gy/fraction for 20 fractions 5 fractions/week. The median follow-up of 74 months shows that this treatment is safe, effective, and well-tolerated, while the duration is shortened, convenient for both patients and the health system [28]. However, the reluctance to use larger radiotherapy fractions to treat the pelvic lymph nodes is not justified as there is an overwhelming experience from established pre-operative rectal cancer regimens delivering 5 Gy for 5 consecutive fractions, with excellent tolerance [29]. In a very recent study, Telkhade et al. [30], treated 60 prostate cancer patients with five fractions of 7 Gy to the prostate and 5 Gy to the nodes, confirming low toxicity and high efficacy.
The HypoAR scheme applied herein to treat pelvic nodes and the prostate area within 14 days is based on an extensive previous experience obtained in our department with a HypoAR schedule delivered postoperatively in various pelvic tumors, including endometrial, bladder, and prostate cancer [31ŌĆō33]. The scheme delivers 14 consecutive fractions of 2.7 Gy to the pelvis, and tolerance and efficacy have been excellent. A booster dose can be safely given to the prostate or bladder, or whatever pelvic tumor using three dimensional (3D) conformal RT, either concurrently or after the 14 fractions. In the current study, the booster dose was delivered simultaneously to the prostate (3.67 Gy) and the seminal vesicles (3.5 Gy) so that the whole therapy was accomplished within 18 days. A VMAT technique was applied that further reduces the dose to critical organs, as compared to the previously applied 3D technique. The choice of 3.67 Gy per fraction is based on a previously tested 3D schedule delivering 15 fractions of 3.5 Gy to the prostate, which gives a biological dose equivalent to the 14 fractions of 3.67 Gy [34].
The radiobiological analysis of the applied HypoAR scheme shows that the late toxicity of bladder, rectum, and sigmoid is lower than a CRT regimen that would deliver the same biological dose to prostate cancer, whether time corrected or not. The parameters that we assumed for our calculations were 2 Gy for ╬▒/╬▓ ratio of prostate tumor, 10 Gy and 4 Gy for early and late effects of normal tissues, respectively. The ╬╗-value for late responding tissues was 0.2 Gy/day. Analysis for early responding tissues (╬▒/╬▓ = 10 Gy) was performed for two extreme ╬╗-values, 0.2 and 0.8 Gy/day. Only for ╬╗-values as high as 0.8 Gy/day predicted for worse early toxicity for HypoAR compared to CRT. The studyŌĆÖs clinical findings showed a very low early toxicity for bladder, rectum, and sigmoid, indicating that ╬╗-values as high as 0.8 Gy/day are not realistic. Although our experience shows that early toxicities expected from HypoAR should be lower than CRT, we assumed equal toxicities to calculate the maximum applicable ╬╗-values. These were 0.61 Gy/day, 0.74 Gy/day and 0.38 Gy/day for bladder, rectum and sigmoid, respectively. It is stressed that, despite the preliminary favorable clinical data, the study is theoretical in its design, having as primary endpoint the radiobiological analysis, and thorough clinical evaluation in a large sample of patients is demanded. Assessment of late toxicities demand longer follow-up and so does the evaluation of the efficacy of the regimen. Although the current study assumes an equal NTD to prostate cancer cells between HypoAR and CRT, based an ╬▒/╬▓ and ╬╗-value assumptions, this remains to be proved in non-inferiority randomized trials. Based on the current encouraging evidence, the study has switched to a phase II trial with a primary end-point the long term toxicities and clinical efficacy with a minimum follow-up of 5 years.
We conclude that the 14-fraction HypoAR scheme addressed to the prostate and pelvic nodes for high-risk prostate cancer has good tolerance and efficacy. This regimen is safe for both the early and late effects of radiation on normal tissues in terms of toxicity. It shortens the duration of treatment, which is convenient for busy radiotherapy departments and much more appealing to patients who have to travel to the radiotherapy departments for 14 instead of 40 days. The question of an eventual superiority in terms of efficacy compared to CRT should be tested in randomized trials.
NotesStatement of Ethics The Institute Ethics and Research Committee of the University Hospital of Alexandroupolis has approved the study (No. ES10 24-10-2018). Author Contributions Conceptualization, CN, MIK. Investigation and methodology, CN, MIK. Supervision, CN, MIK. Writing of the original draft, CN. Writing of the review and editing, MIK, VS, AZ. Validation, MIK, VS, AZ. Formal analysis, CN, MIK. Data curation, CN, MIK. All the authors have proofread the final version. Fig.┬Ā1.Dose distributions for each PTV: (A) PTV prostate, (B) PTV seminal vesicles and (C) PTV pelvic lymph nodes. In each graph the absolute doses (in cGy) represent the relative isodoses of 100%, 95%, 90%, 75%, and 50%, respectively. PTV, planning target volume. 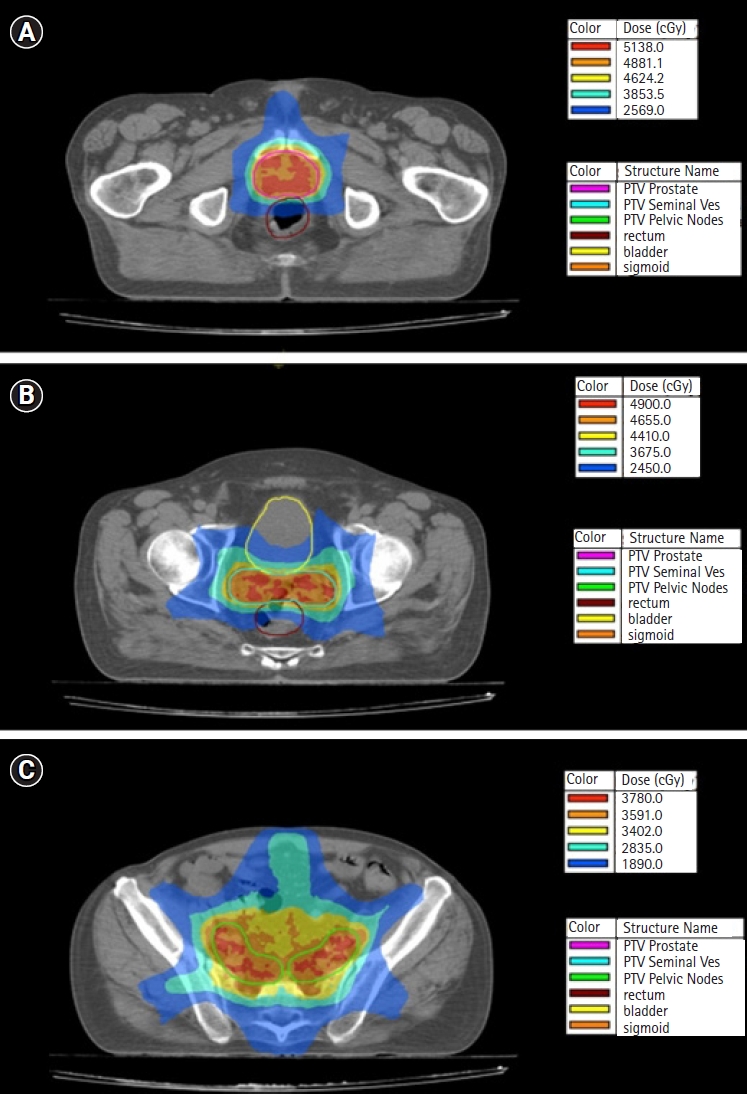
Fig.┬Ā2.Comparison of four points (D50%, D35%, D25%, D15%) of average bladder, rectum and sigmoid dose-volume histogram corrected for fractionation related to early toxicities (╬▒/╬▓ = 10 Gy, ╬╗ = 0.2 Gy/day and 0.8 Gy/day) for HypoAR and CRT without time correction (AŌĆōC) and with time correction (DŌĆōF) and (GŌĆōI). NTD, normalized total dose without time correction; NTD_T, normalized total dose with time correction; HypoAR, hypofractionated and accelerated radiotherapy; CRT, conventionally radiotherapy. 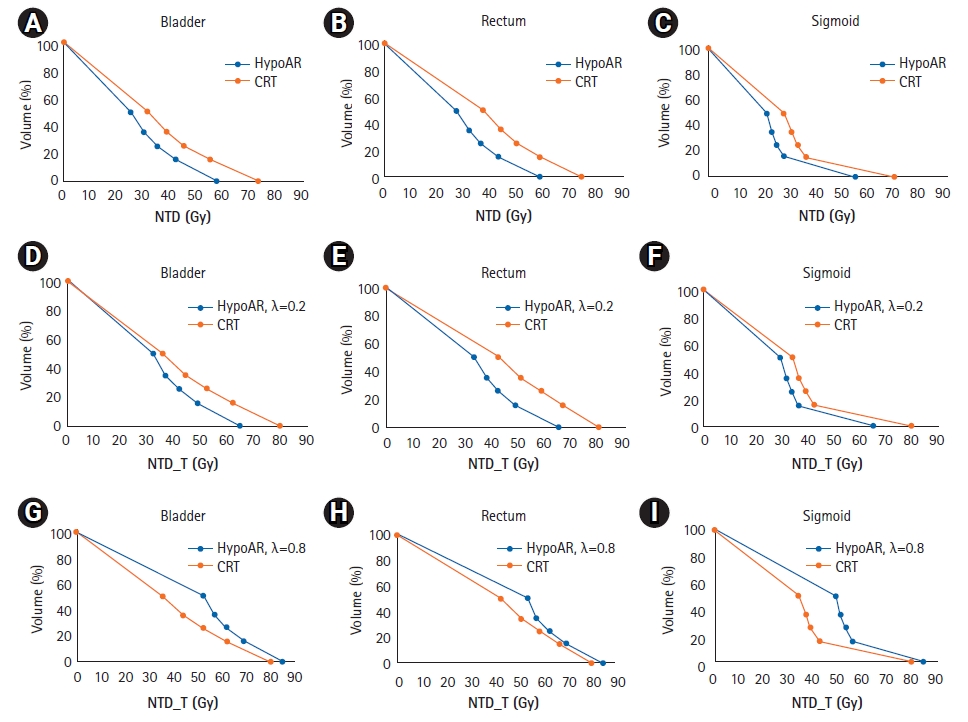
Fig.┬Ā3.Comparison of four points (D50%, D35%, D25%, D15%) of average bladder, rectum and sigmoid dose-volume histogram corrected for fractionation related to late toxicities (╬▒/╬▓ = 4 Gy, ╬╗ = 0.2 Gy/day) for HypoAR and CRT without time correction (A, C, E) and with time correction (B, D, F). NTD, normalized total dose without time correction; NTD_T, normalized total dose with time correction; HypoAR, hypofractionated and accelerated radiotherapy; CRT, conventionally radiotherapy. 
Table┬Ā1.Patient and disease characteristics (n = 22)
Table┬Ā2.Comparison of average NTD and NTD_T received by early responding tissue components of the bladder, rectum and sigmoid (╬▒/╬▓ = 10 Gy), calculated at 50%, 35%, 25% and 15% of organ volumes (for ╬▒/╬▓ = 10 Gy, ╬╗ = 0.2 Gy/day), between HypoAR and CRT delivering the same NTD and NTD_T to the prostate cancer (╬▒/╬▓ = 2 Gy) Table┬Ā3.Comparison of average NTD_T received by early responding tissue components of the bladder, rectum and sigmoid (╬▒/╬▓ = 10 Gy), calculated at 50%, 35%, 25% and 15% of organ volumes (for ╬▒/╬▓ = 10 Gy, ╬╗ = 0.8 Gy/day), between HypoAR and CRT delivering the same NTD_T to the prostate cancer (╬▒/╬▓ = 2 Gy) Table┬Ā4.Comparison of average NTD and NTD_T received by late responding tissue components of the bladder, rectum and sigmoid (╬▒/╬▓ = 4 Gy), calculated at 50%, 35%, 25% and 15% of organ volumes (for ╬▒/╬▓ = 4 Gy, ╬╗ = 0.2 Gy/day), between HypoAR and CRT delivering the same NTD and NTD_T to the prostate cancer (╬▒/╬▓ = 2 Gy) References1. Di Franco R, Borzillo V, Ravo V, et al. Rectal/urinary toxicity after hypofractionated vs. conventional radiotherapy in high risk prostate cancer: systematic review and meta analysis. Eur Rev Med Pharmacol Sci 2017;21:3563ŌĆō75.
2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018 [cited 2022 May 30]. Available from: www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
3. Aas K, Berge V, Myklebust TA, Fossa SD. Comparative survival outcomes of high-risk prostate cancer treated with radical prostatectomy or definitive radiotherapy regimens. Eur Urol Open Sci 2021;26:55ŌĆō63.
4. Memorial Sloan Kettering Cancer Center (MSKCC) Nomogram: probability of lymph node involvement in prostate cancer patients (includes biopsy cores) [Internet]. Haaksbergen, The Netherlands: Evidencio; c2022 [cited 2022 May 30]. Available: https://www.evidencio.com/models/show/440.
5. Murthy V, Maitre P, Kannan S, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): outcomes from phase III randomized controlled trial. J Clin Oncol 2021;39:1234ŌĆō42.
6. Murthy V, Maitre P, Bhatia J, et al. Late toxicity and quality of life with prostate only or whole pelvic radiation therapy in high risk prostate cancer (POP-RT): a randomised trial. Radiother Oncol 2020;145:71ŌĆō80.
7. Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6ŌĆō13.
8. Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol 2018;36:JCO1801097.
9. Magli A, Moretti E, Tullio A, et al. Hypofractionated simultaneous integrated boost (IMRT-SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis 2018;21:269ŌĆō76.
10. Hernandez TG, Gonzalez AV, Peidro JP, et al. Radiobiological comparison of two radiotherapy treatment techniques for high-risk prostate cancer. Rep Pract Oncol Radiother 2013;18:265ŌĆō71.
11. Maciejewski B, Taylor JM, Withers HR. Alpha/beta value and the importance of size of dose per fraction for late complications in the supraglottic larynx. Radiother Oncol 1986;7:323ŌĆō6.
12. Koukourakis MI, Damilakis J. LQ-based model for biological radiotherapy planning. Med Dosim 1994;19:269ŌĆō77.
13. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: ╬▒/╬▓ = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys 2012;82:e17ŌĆō24.
14. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095ŌĆō101.
15. Brenner D, Armour E, Corry P, Hall E. Sublethal damage repair times for a late-responding tissue relevant to brachytherapy (and external-beam radiotherapy): implications for new brachytherapy protocols. Int J Radiat Oncol Biol Phys 1998;41:135ŌĆō8.
16. Deore SM, Shrivastava SK, Supe SJ, Viswanathan PS, Dinshaw KA. Alpha/beta value and importance of dose per fraction for the late rectal and recto-sigmoid complications. Strahlenther Onkol 1993;169:521ŌĆō6.
17. Tucker SL, Thames HD, Michalski JM, et al. Estimation of ╬▒/╬▓ for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys 2011;81:600ŌĆō5.
18. Thames HD, Kuban D, Levy LB, et al. The role of overall treatment time in the outcome of radiotherapy of prostate cancer: an analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol 2010;96:6ŌĆō12.
19. Bentzen SM, Saunders MI, Dische S. From CHART to CHARTWEL in non-small cell lung cancer: clinical radiobiological modelling of the expected change in outcome. Clin Oncol (R Coll Radiol) 2002;14:372ŌĆō81.
20. Bentzen SM, Skoczylas JZ, Bernier J. Quantitative clinical radiobiology of early and late lung reactions. Int J Radiat Biol 2000;76:453ŌĆō62.
21. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1ŌĆō10.
22. Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S10ŌĆō9.
23. Da Pozzo LF, Cozzarini C, Briganti A, et al. Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol 2009;55:1003ŌĆō11.
24. Dearnaley D, Griffin CL, Lewis R, et al. Toxicity and patient-reported outcomes of a phase 2 randomized trial of prostate and pelvic lymph node versus prostate only radiotherapy in advanced localised prostate cancer (PIVOTAL). Int J Radiat Oncol Biol Phys 2019;103:605ŌĆō17.
25. Adkison JB, McHaffie DR, Bentzen SM, et al. Phase I trial of pelvic nodal dose escalation with hypofractionated IMRT for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:184ŌĆō90.
26. Ishii K, Ogino R, Hosokawa Y, et al. Whole-pelvic volumetric-modulated arc therapy for high-risk prostate cancer: treatment planning and acute toxicity. J Radiat Res 2015;56:141ŌĆō50.
27. Sashidharan S, Beena K, Madhavan R, Menon D, Makuny D. EP-1362: Hypofractionated simultaneous integrated boost IMRT in high risk prostate cancer: a novel approach. Radiot Oncol 2016;119:S636.
28. Faria S, Ruo R, Perna M, et al. Long-term results of moderate hypofractionation to prostate and pelvic nodes plus androgen suppression in high-risk prostate cancer. Pract Radiat Oncol 2020;10:e514.
29. Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336ŌĆō46.
30. Telkhade T, Murthy V, Kanala TS, et al. Safety and efficacy of ultra-hypofractionation in node-positive prostate cancer. Clin Oncol (R Coll Radiol) 2021;33:172ŌĆō80.
31. Panteliadou M, Giatromanolaki A, Touloupidis S, et al. Treatment of invasive bladder cancer with conformal hypofractionated accelerated radiotherapy and amifostine (HypoARC). Urol Oncol 2012;30:813ŌĆō20.
32. Koukourakis MI, Kyrgias G, Panteliadou M, et al. Dose escalation of amifostine for radioprotection during pelvic accelerated radiotherapy. Am J Clin Oncol 2013;36:338ŌĆō43.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |