 |
 |
AbstractPurposeTo compare biochemical recurrence-free survival (BRFS) and toxicity outcomes of high dose rate brachytherapy (HDRB) and stereotactic body radiotherapy (SBRT) boost after elective nodal irradiation for high/very high-risk prostate cancer.
Materials and MethodsA retrospective analysis was performed in 149 male patients. In 98 patients, the boost to the prostate was delivered by HDRB as 2 fractions of 10 Gy (EQD2 for α/β = 1.5; 66 Gy) or 1 fraction of 15 Gy (EQD2 for α/β = 1.5; 71 Gy). In 51 male patients, SBRT was used for the boost delivery (3 fractions of 7 Gy; EQD2 for α/β = 1.5; 51 Gy) because brachytherapy equipment was out of order.
ResultsIn 98 patients that received HDRB boost, 3- and 5-year BRFS were 74.6% and 66.8%. Late grade-II genitourinary toxicity was detected in 27, grade-III in 1 case. Grade-II (maximum) rectal toxicity was diagnosed in nine patients. For 51 male patients that received SBRT boost, 3- and 5-year BRFS was 76.5% and 67.7%. Late grade-II (maximum) genitourinary toxicity was detected in five cases, late grade-II rectal toxicity in four cases. Other three patients developed late grade-III–IV rectal toxicity that required diverting colostomy. SBRT boost was associated with higher maximum dose to 2 cm3 of anterior rectal wall (D2cm³rectum) compared to HDRB: 92% versus 55% of dose to prostate. Severe rectal toxicity was negligible at EQD2 D2cm³rectum <85 Gy and EQD2 D5cm³ rectum <75 Gy.
IntroductionRadiotherapy is established as a standard radical treatment for patients with localized prostate cancer. The results of prospective randomized studies confirmed that radiotherapy in low and intermediate risk patients is as effective as a surgical treatment while providing a better safety profile [1]. Separate retrospective studies confirm that in male with high and very high-risk prostate cancer surgery is associated with higher rates of overall and cancer specific survival compared to the external beam radiotherapy [2,3]. However, recently several doses escalated studies that utilized high dose rate brachytherapy (HDRB) boost in addition to pelvic irradiation outperformed surgical treatment and external beam radiotherapy in terms of biochemical control, distant metastatic free and/or cancer specific survival [4-7]. The studies mentioned above validate the use of brachytherapy as the standard method for prostate boost as it allows delivery of highest total equivalent dose (EQD2 >100 Gy) without a significant increase of radiation burden for normal tissues [5,6,8].
Accumulated clinical experience confirms that stereotactic ablative body radiotherapy (SBRT) as well as brachytherapy is characterized by highly accurate irradiation of the prostate with sharp dose gradient and efficient sparing of surrounding normal tissues [9,10]. This makes it possible to consider SBRT as a promising non-invasive method of boost delivery to the prostate. However, there are no clinical data with direct comparison of the efficacy and safety of HDRB versus SBRT boost in treatment of high and very high-risk prostate cancer. Therefore, in this single center retrospective study we analyzed our personal experience of using HDRB and SBRT for delivery of the boost to the prostate after elective pelvic nodal irradiation.
Materials and MethodsIn the practice of the Institute, HDRB has been used since 2012 for monotherapy of low and intermediate risk prostate cancer, and as a routine technique for boost delivery after pelvic nodal irradiation in high and very high-risk groups. SBRT has been used since 2013 for monotherapy of patients with low and intermediate risk prostate cancer. Unfortunately, our brachytherapy equipment was out of order twice for periods of 145 and 55 days. At this time SBRT was the only modality for “boosting” the prostate (33 cases). In addition, since 2016 in patients with contraindications for spinal anesthesia and/or severe comorbidities we also have used SBRT for “boost” delivery (18 cases).
Finally, 149 patients with high/very high-risk prostate cancer (clinical stage T3–4, Gleason >7, prostate specific antigen [PSA] >20 ng/mL) treated at N.N. Petrov National Medical Research Center of Oncology between June 2012 and April 2018 were included in the analysis approved by the local Ethical Committee (No. 09/2020). Written informed consent was obtained from all participants before the start of the treatment. One-third (31.7%) of the patients had instrumental signs (MRI, PET-CT) of pelvic lymph node involvement. Staging was performed according to the American Joint Committee on Cancer (AJCC) 7th edition. All male patients had androgen deprivation therapy with subsequent pelvic lymph node irradiation initiated 1–3 months after the start of hormonal treatment: in 93 patients as 3D conformal 4-field technique; in the remaining 56 male patients using volumetric modulated arc therapy (VMAT)—the prescribed dose varied from 43 Gy to 50 Gy (Table 1).
1. Brachytherapy boostFrom June 2012 to June 2017. 98 patients (average age, 65.1 years; range, 49 to 82 years) received brachytherapy boost delivered as 2 fractions (2 implantations) of 10 Gy (81 males) or 1 fraction of 15 Gy (17 males) 3–4 weeks after the end of pelvic irradiation. The mean intervals between fractions was 18.4 days (range, 14 to 24 days) and never exceed 24 days. Total equivalent dose in 2 Gy fractions (EQD2) of the boost were 66–71 Gy (EQD2 for α/β = 1.5) and 52–71 Gy (EQD2 for α/β = 3).
Implantation of the needles was performed under spinal anesthesia with transrectal ultrasound (TRUS) guidance. Pre- and post-implantation automatic 3D TRUS image acquisition was performed for real-time intraoperative treatment planning with inverse and/or volumetric optimization algorithms using Oncentra Prostate planning system (Elekta, Stockholm, Sweden). The organs-at-risk defined in every case were as follows: anterior rectal wall, urethra (catheter), bladder neck (catheter balloon). Gross tumour volume (GTV) was defined as the prostate and proximal 1/2–2/3 of seminal vesicles; clinical target volume (CTV) was created by 2–3 mm expansion of GTV in all directions except posterior and in the direction of the bladder. The dose was prescribed to CTV with the following objectives: CTV V100 >93%, CTV D90 >100%; urethra maximum dose <115% and urethra D10 <110%. A 2 cm3 maximum dose to the bladder neck and anterior rectal wall should be kept below 75% (D2cm3 <75%).
2. SBRT boostFrom May 2015 to April 2018, 51 patients (mean age, 68.4 years; range, 51 to 76 years) received SBRT boost to the prostate: in 33 cases as the substitute of HDRB because brachytherapy equipment was out of order, and in the remaining 18 patients because of contraindication for spinal anesthesia. Patient characteristics are presented in Table 1. The boost was delivered as 3 fractions of 7 Gy each (51 Gy EQD2 for α/β = 1.5 and 42 Gy EQD2 for α/β = 3). Gold fiducial markers (3 per patient) were transperineally inserted into the prostate under the TRUS guidance. Planning MRI and simulation CT were performed with an empty rectum and a full (200–400 mL) bladder 3–7 days after fiducial markers placement. GTV and CTV were the same as described earlier for HDRB boost, and planning target volume (PTV) was 3–5 mm in all directions and 1–3 mm posteriorly. The dose was prescribed to 90% PTV. A total dose of 21 Gy in 3 daily fractions was delivered with TrueBeam or Novalis (Varian Medical Systems, Palo Alto, CA, USA) linear accelerators. Cone-beam CT was used for treatment guidance before and after each VMAT session. In male with full rectum and/or empty bladder SBRT session was delayed and performed after adequate preparation of the patient. Spacers or rectal balloons were not used in patients included in the analysis.
3. Patient follow-up and toxicity assessmentAccording to our standard follow-up strategy, patients were evaluated every 3 months for the first year and every 6 months thereafter. The primary endpoints of this analysis were biochemical disease-free survival and late toxicity. Acute toxicity and late toxicity were determined using Common Terminology Criteria for Adverse Events (CTCAE) version 5 [11]. For each person the highest CTCAE score recorded 6 and more months after the end of treatment was defined as maximum late toxicity.
PSA was obtained at baseline, every 3 months for the first 2 years and every 6 months thereafter. The PSA relapse was diagnosed as nadir after the end of treatment plus 2 ng/mL (Phoenix definition). PSA bounce was characterized by PSA raised above nadir with a subsequent decline to nadir level.
The correlations of treatment (HDRB vs. SBRT) and dosimetric variables with the probability of severe late rectal complications were analyzed with χ2 test and t-test for independent variables. Biochemical relapse-free survival was evaluated using Kaplan-Meier curves. A p-values below 0.05 were considered significant.
ResultsThe median follow-up in the group of HDRB boost was 87.7 months (range, 42.1 to 102.0 months). The 3-year and 5-year BRFS were 75.6% and 66.8%, respectively (Fig. 1).
During the follow-up period, one patient died of prostate cancer; three patients died of other reasons (cardiovascular decompensation, second tumor). Local recurrences in the prostate were detected in 3 (10.3%) of 29 patients with biochemical relapse. Pelvic nodes involvements were detected in 8 (27.6%) cases, retroperitoneal lymph nodes above the bifurcation of aorta in other 11 (37.9%) observations. Bone metastases were diagnosed in 11 (37.9%) of 29 patients with biochemical relapse.
Late grade III urinary toxicity was detected in 1 (1.1%) of 98 patients. It manifested by urethra stricture surgically treated with a good functional result. Grade II toxicity was mentioned in 28 (28.6%) cases and in most observations (24 male) manifested by frequency and/or urgency that were successfully treated by anti-inflammatory therapy and ɑ-blockers. Grade II rectal toxicity was detected in 8 (8.2%) cases and manifested by episodes of rectal bleeding and/or painful defecation that were the cause of conservative treatment. In one man, we diagnosed a subcompensated rectal stenosis treated conservatively and also estimated as grade II toxicity.
In 51 patients who received SBRT boost, the median follow-up was 55.1 months (range, 31.1 to 69.1 months); two patients were lost for follow-up 38 and 43 months after the end of treatment. The 3-year biochemical relapse-free survival was 82.3%, 5-year was 67.7%. Biochemical relapses were diagnosed in 12 patients: in 8 of 12 cases they manifested by bone metastases and were subclinical in the remained four observations.
Late grade II urinary complications (two cases of urinary urgency and/or frequency and three cases of retention treated by ɑ-blockers) were detected in five patients (9.8%). We explained lower incidents of grade II urinary toxicity after SBRT by non-invasive character of this technique that does not require catheterization of urethra and needle insertion. Late grade II rectal toxicity was detected in four cases (8.6%) and in all observations was represented by rectal bleedings (2 males) that did not recur, and/or painful defecation (2 males) that were the cause of conservative treatment.
Late grade III–IV rectal toxicity was revealed in three cases (5.9%). In three patients with proctitis and clinically significant bleedings the conservative treatment by argon plasma coagulation and hemotransfusions was ineffective. In all three cases the patients underwent colostomy, one male patient that was on oral anticoagulant after the coronary surgery, manifested by severe bleeding and was treated in the intensive care unit (grade IV toxicity).
Taking into account significantly higher risk of grade III–IV rectal toxicity in patients who received SBRT boost to the prostate (p = 0.038), we performed dosimetric comparison of HDRB and SBRT treatment plans (Table 2, Fig. 2). The presented data demonstrated two important dosimetric differences between HDRB and SBRT boost: significantly lower dose to the anterior rectum wall (p < 0.0001) and higher mean prostate dose (p < 0.001) in patients who received HDRB boost. In patients who received SBRT boost, we separately analyzed the correlation between doses absorbed by anterior rectum wall (D2cm3 and D5cm3) and probability of severe rectal toxicity (Tables 3, 4). According to our data, the risk of severe late rectal toxicity is negligible when D2cm3 and D5cm3 (ɑ/β = 3) are below 85 Gy and 75 Gy, respectively. The probability of late rectal complication becomes unacceptable when D2cm³ and D5cm3 (ɑ/β = 3) are above 90 Gy and 81 Gy.
Discussion and ConclusionThe performed retrospective analysis demonstrates a similar efficacy of HDRB and SBRT when used as a prostate boost after regional lymph node irradiation in combination with hormonal therapy: 3-year and 5-year biochemical relapse-free survival was 74.6% versus 76.5% and 66.8% versus 67.7%, respectively. Literature data demonstrated a substantial variability of BRFS rates for patients with high and/or very high-risk prostate cancer. Some authors reported that it was in the range of 81%–91% even after the monotherapy with HDRB or SBRT [12,13]. These data corresponded to our previous findings of 78% of BRFS after HDRB of patients with high-risk prostate cancer [14]. On the other hand, the prospective multicenter trials and some retrospective reports pointed out that 5-year BRFS in mixed population of high and very high-risk prostate cancer patients varied from 47% to 67% [6,15,16]. Taking into account the predominance of very high-risk patients in both HDRB and SBRT boost groups of our study and considering that most of relapses were represented by generalization with bone and/or “out of fields” lymph node metastases, the presented rates of BRFS seem acceptable. It is also important to mention very high rates of a 5-year locoregional control that exceeded 90% in HDRB group, and was equal to 100% after a 3-year follow-up in male who underwent SBRT boost.
The biggest surprise of this study was the high level of severe late rectal toxicity after SBRT boost. Our previous experience with HDRB, SBRT monotherapy and HDRB boost in more than 900 patients with prostate cancer indicates excellent safety profile for both procedures with grade III rectal toxicity below 1%. In patients who received HDRB boost, we did not mentioned grade III rectal toxicity, and late grade II toxicity was detected only in 8.2% cases. The dosimetric analysis gives some explanations to the observed differences: D0.1cm3 and D2cm3 for anterior rectal wall was about 22%–28% higher with SBRT boost. These dosimetric differences in the dose to the anterior rectal wall were already mentioned in the literature [17-19]. In particular, in the study of Frohlich et al. [19], D2cm3 to the rectum was 1.8 higher with SBRT compared to HDRB. Literature analysis clearly demonstrated that these differences transformed into important clinical consequences. For instance, it was shown that low and high dose rate brachytherapy was associated with significant reduction of grade III rectal toxicity when compared to external beam radiotherapy [4,20,21], SBRT [22] as monotherapy treatment and SBRT as a boost [18,23]. It is interesting that in all of these studies brachytherapy manifested by maximum grade II rectal toxicity that matches our data. Conversely, grade III rectal late toxicity varied in most of these studies around 2.5%-5% [4,18,22,23]. Parzen et al. [21] analyzed rectal toxicity in 2,863 patients with prostate cancer and mentioned that compared to external beam radiotherapy HDRB was associated with the decreased rates of chronic rectal grade ≥ II bleeding (1.3% vs. 8.7%).
Dose escalation to the primary tumor in high/very high-risk prostate cancer is an important factor associated with high rates of long-term relapse free and overall survival [24,25]. On the other hand, reduction of radiation dose to the anterior rectal wall below its tolerance minimized the risk of severe functional impairments [26,27]. When choosing the technique of SBRT boost (total dose and fractionation scheme), we were guided by numerous reports on maximum tolerant doses to the anterior rectal wall that correspond to acceptable (≤1%–2%) severe (grade ≥ III) rectal toxicity in monotherapy [27-29] or boost [30-32] trials. In SBRT studies it was shown that the EQD2 for α/β = 3 to 2 cm3 of anterior rectal wall in the range of 83.5–92.5 Gy was associated with minimal severe toxicity [22,23,28-30]. A comprehensive analysis of rectal toxicity in dose escalation study was performed by Kim et al. [26], and the authors concluded that delayed severe rectal injury occurs when >3 cm3 of continues rectal wall received 50 Gy (EQD2 for α/β = 3 ≥130 Gy). The authors also postulated three main mechanisms of rectal toxicity: direct stem cell inactivation, disrupted stem cell migration and vascular/stroma damage which is closely correlated with the delayed rectal wall tolerance [26].
Our practice with SBRT boost indicated a significantly lower threshold dose in terms of late rectal toxicity. In particular, we found out that it is possible to minimize the probability of severe late rectal toxicity when D2cm3 and D5cm3 (ɑ/β = 3) are below 85 Gy and 75 Gy. Probably, such differences in tolerance doses can be explained by a more aggressive treatment of our patients—they received a combination of pelvic lymph node irradiation with SBRT. In this case, the whole circumfluence of the rectum received 46–50 Gy with additional dose to the anterior rectal wall during the delivery of the boost. Another possible explanation is the aggressive local treatment of the rectal bleeding—all three males with grade III rectal toxicity underwent endoscopic invasive local interventions in non-oncologic outpatient clinics.
To our knowledge, this is the first study that directly compares HDRB and SBRT when used as a boost in combination with a standard elective pelvic nodal irradiation, but our results have several important limitations. The main two are a relatively short follow-up period, especially in SBRT boost group, and the absence of the true randomization. It is well known that even in patients with high and very high prostate cancer many biochemical and clinical relapses can manifest after 5–10 years of follow-up. On the other hand, most events related to late toxicity become evident during the first 3 years after the treatment, and it can be proposed that the observation time was sufficient to capture most of the late treatment complications. The retrospective nature of this analysis compromises the comparison of two boost techniques. On the other hand, taking into account that indications for combined treatment in both groups were identical, and the choice of boost technique was driven only by the availability of HDRB equipment, it seems that the presented results objectively reflect the differences in efficacy and safety of both boost strategies.
In conclusion, our results indicate similar 3-year and 5-year biochemical control in patients with high/very high-risk prostate cancer who received elective pelvic irradiation with HDRB or SBRT boost. We also found out that SBRT boost was associated with higher late rectal toxicity than HDRB boost. The dosimetric comparison of SBRT and HDRB demonstrates that HDRB can deliver the prescribed dose to the prostate with a significantly lower exposure (28% lower D2cm3) to the anterior rectal wall. Our data show that in patients who received SBRT boost, the risk of severe late toxicity occurs when the dose absorbed by anterior rectal wall exceeds D2cm3 (ɑ/β = 3) > 85 Gy and/or D5cm3 (ɑ/β = 3) >75 Gy.
NotesAuthor Contributions Conceptualization: SNN, SVK; Funding acquisition: SNN, RVN; Investigation and methodology: SNN, RVN, YOM, MYG, NDI, YSM; Project administration: SNN, SVK; Resources: SNN, SVK; Supervision: SNN, RVN; Writing of the original draft: SNN, RVN, YOM, MYG, NDI, YSM; Writing of the review and editing: SNN; Software: RVN, YOM; Validation: SNN; Formal analysis: SNN, RVN, SVK; Data curation: SNN, RVN, YOM, MYG, NDI, YSM; Visualization: SNN, RVN, YOM. All the authors have proofread the final version. Fig. 1.Biochemical relapse-free survival in patients with HDRB (blue) or SBRT (red) boost to the prostate. HDRB, high dose rate brachytherapy; SBRT, stereotactic body radiotherapy. 
Fig. 2.Axial views of the US-based trans-rectal HDRBT and SBRT plans with overlaid dose distribution. (A) Axial view of the US-based HDRB plan. Red line indicates contour of prostate; dark blue line, contour of anterior rectal wall; yellow line, 100% isodose; light cyan line, 80% isodose (white arrow); dark cyan line, 50% isodose (yellow arrow). Only 50% isodose cross small volume of anterior rectal wall. (B) Axial CT-MRI fused image of the prostate with one visualized fiducial in the right lobe. Treatment plan of the prostate SBRT. Red line indicates prostate and CTV contours; magenta line, contour of the rectum; green line, 100% isodose; dark blue line, 80% isodose (white arrow); cyan line, 50% isodose (yellow arrow). Half of the rectum volume is covered by 50% isodose, the whole anterior rectum wall is covered by 80% isodose, 100% isodose “touch” anterior rectal wall. SBRT, stereotactic body radiotherapy; HDRB, high dose rate brachytherapy; US, ultrasound; CT, computerized tomography; MRI, magnetic resonance tomography. 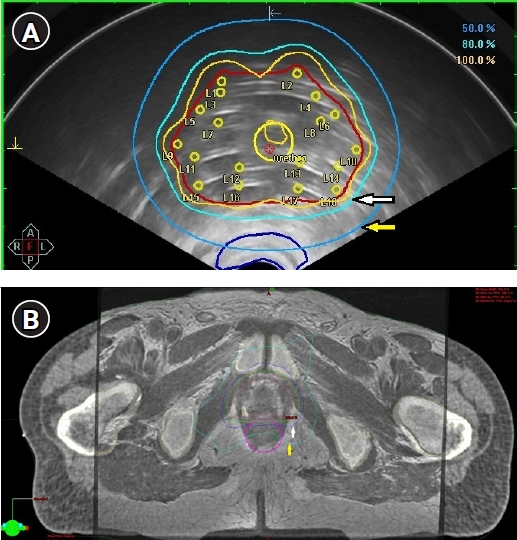
Table 1.Patient and tumor characteristics Table 2.Dosimetric parameter comparison Values are presented as mean ± standard deviation. Doses represented as the percent of the prescription dose. HDRB, high dose rate brachytherapy; SBRT, stereotactic body radiotherapy; V100%, volume of the prostate (gross tumor volume) receiving 100% of the prescription dose; D90%, radiation dose delivered to 90% of the prostate (gross tumor volume); D0.1cm3, maximum dose received by 0.1 cm3 of the anterior rectal wall; D2cm3, maximum dose received by 2 cm3 of the anterior rectal wall; D10%, dose received by 10% of the urethra volume. Table 3.Late gastrointestinal toxicity in accordance with the maximum dose to the 2 cm3 of anterior rectal wall
Table 4.Late gastrointestinal toxicity in accordance with the maximum dose to the 5 cm3 of anterior rectal wall
References1. Neal DE, Metcalfe C, Donovan JL, et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the protect randomised controlled trial according to treatment received. Eur Urol 2020;77:320–30.
2. Knipper S, Palumbo C, Pecoraro A, et al. Survival outcomes of radical prostatectomy vs. external beam radiation therapy in prostate cancer patients with Gleason score 9-10 at biopsy: a population-based analysis. Urol Oncol 2020;38:79.
3. Chierigo F, Wenzel M, Wurnschimmel C, et al. Survival after radical prostatectomy versus radiation therapy in high-risk and very high-risk prostate cancer. J Urol 2022;207:375–84.
4. Ciezki JP, Weller M, Reddy CA, et al. A comparison between low-dose-rate brachytherapy with or without androgen deprivation, external beam radiation therapy with or without androgen deprivation, and radical prostatectomy with or without adjuvant or salvage radiation therapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;97:962–75.
5. Kishan AU, Cook RR, Ciezki JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with Gleason score 9-10 prostate cancer. JAMA 2018;319:896–905.
6. Sandler KA, Cook RR, Ciezki JP, et al. Clinical outcomes for patients with Gleason score 10 prostate adenocarcinoma: results from a multi-institutional consortium study. Int J Radiat Oncol Biol Phys 2018;101:883–8.
7. Yin M, Zhao J, Monk P, et al. Comparative effectiveness of surgery versus external beam radiation with/without brachytherapy in high-risk localized prostate cancer. Cancer Med 2020;9:27–34.
8. Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012;103:217–22.
9. Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys 2019;104:778–89.
10. Lehrer EJ, Kishan AU, Yu JB, et al. Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: a systematic review and meta-analysis of phase III randomized trials. Radiother Oncol 2020;148:235–42.
11. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [Internet]. Washington, DC: US Department of Health and Human Services; 2017 [cited 2022 Sep 17]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
12. Gonzalez-Motta A, Roach M. Stereotactic body radiation therapy (SBRT) for high-risk prostate cancer: where are we now? Pract Radiat Oncol 2018;8:185–202.
13. Viani GA, Arruda CV, Assis Pellizzon AC, De Fendi LI. HDR brachytherapy as monotherapy for prostate cancer: a systematic review with meta-analysis. Brachytherapy 2021;20:307–14.
14. Novikov S, Kanaev S, Novikov R, Ilin N, Gotovchikova M, Girshovitch M. HDR brachytherapy as monotherapy or a boost for high risk prostate cancer: 5 year single center data [Internet]. Brussels, Belgium: European Society for Radiotherapy and Oncology; 2021 [cited 2022 Sep 17]. Available from: https://www.estro.org/Congresses/WCB-2021/456/poster-prostate/3265/hdrbrachytherapyasmonotherapyoraboostforhighriskpr.
15. Vargas C, Martinez A, Galalae R, et al. High-dose radiation employing external beam radiotherapy and high-dose rate brachytherapy with and without neoadjuvant androgen deprivation for prostate cancer patients with intermediate- and high-risk features. Prostate Cancer Prostatic Dis 2006;9:245–53.
16. Roach M, Moughan J, Lawton CA, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol 2018;19:1504–15.
17. Chatzikonstantinou G, Keller C, Scherf C, Bathen B, Kohn J, Tselis N. Real-world dosimetric comparison between CyberKnife SBRT and HDR brachytherapy for the treatment of prostate cancer. Brachytherapy 2021;20:44–9.
18. Sanmamed N, Lee J, Berlin A, et al. Tumor-targeted dose escalation for localized prostate cancer using MR-guided HDR brachytherapy (HDR) or integrated VMAT (IB-VMAT) boost: dosimetry, toxicity and health related quality of life. Radiother Oncol 2020;149:240–5.
19. Frohlich G, Agoston P, Jorgo K, Stelczer G, Polgar C, Major T. Comparative dosimetrical analysis of intensity-modulated arc therapy, CyberKnife therapy and image-guided interstitial HDR and LDR brachytherapy of low risk prostate cancer. Rep Pract Oncol Radiother 2021;26:196–202.
20. Buchser D, Casquero F, Espinosa JM, et al. Late toxicity after single dose HDR prostate brachytherapy and EBRT for localized prostate cancer: clinical and dosimetric predictors in a prospective cohort study. Radiother Oncol 2019;135:13–8.
21. Parzen JS, Ye H, Gustafson G, et al. Rates of rectal toxicity in patients treated with high dose rate brachytherapy as monotherapy compared to dose-escalated external beam radiation therapy for localized prostate cancer. Radiother Oncol 2020;147:123–9.
22. Gogineni E, Rana Z, Soberman D, et al. Biochemical control and toxicity outcomes of stereotactic body radiation therapy versus low-dose-rate brachytherapy in the treatment of low-and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2021;109:1232–42.
23. Chen WC, Li Y, Lazar A, et al. Stereotactic body radiation therapy and high-dose-rate brachytherapy boost in combination with intensity modulated radiation therapy for localized prostate cancer: a single-institution propensity score matched analysis. Int J Radiat Oncol Biol Phys 2021;110:429–37.
24. Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:363–70.
25. Levin-Epstein RG, Jiang NY, Wang X, et al. Dose-response with stereotactic body radiotherapy for prostate cancer: a multi-institutional analysis of prostate-specific antigen kinetics and biochemical control. Radiother Oncol 2021;154:207–13.
26. Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;89:509–17.
27. Wang K, Chen RC, Kane BL, et al. Patient and dosimetric predictors of genitourinary and bowel quality of life after prostate SBRT: secondary analysis of a multi-institutional trial. Int J Radiat Oncol Biol Phys 2018;102:1430–7.
28. Paydar I, Pepin A, Cyr RA, et al. Intensity-modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: a report on 3-year toxicity. Front Oncol 2017;7:5.
29. Zelefsky MJ, Kollmeier M, McBride S, et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2019;104:42–9.
30. Lin YW, Lin LC, Lin KL. The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Front Oncol 2014;4:278.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |