 |
 |
AbstractPigmented villonodular synovitis (PVNS) is a proliferative, recurrent and locally invasive disease of the synovium. The symptoms of the disorder are not typical and thus it is very often misdiagnosed. Most of the times, magnetic resonance imaging presents the nodular model of development and sets the basis for the diagnosis. The final diagnosis will be set by the pathological evaluation of the lesion’s biopsy. PVNS may be localized (nodule with a clear boundary with/without presence of single pedicle) or diffuse (extensive involvement of the adjacent nerves and vessels). Depending on the extension of the PVNS, a different management approach is performed, lesion excision vs. resection, followed by radiotherapy respectively. We report a case of diffuse PVNS in the knee joint, treated with surgical excision and adjuvant radiotherapy as well as follow-up imaging after a time period of 3 years.
IntroductionPigmented villonodular synovitis (PVNS) is a rare, benign, proliferative but potentially locally aggressive, recurrent clinical entity. Usually, it is associated with t(1;2)(CSF-1;COL6A3) chromosomal translocation which results in overexpression of CSF1 (colony stimulating factor 1) [1]. It is characterized by hyperplasia and deposition of pigment (hemosiderin) in the joints and formation of tendon papillae and follicles [2,3]. It occurs mainly between the 3rd and 4th decade of life, but can occur in both children and the elderly [4]. The onset of the disease can be insidious, with symptoms often presenting even years before diagnosis. It mainly affects large joints such as the knee, hip and ankle joints [1,5]. Depending on the biological behavior, PVNS is divided into two different forms. These are localized and diffuse and despite the fact that they present the same histopathological features, they also differ (except for the biological behavior) in management and prognosis [1,6].
Hantes et al. [7] showed that simple total excision of the lesion is the golden standard for the treatment of localized PVNS offering a long-term period of no recurrence [8]. Arthroscopic resection is mainly recommended for localized PVNS [7], while open arthrotomy and total synovectomy is the treatment of choice for the extensive, extra-articular diffuse PVNS. Diffuse PVNS is difficult to manage due to its extent, especially when nearby structures are involved. The recurrence rate ranges from 14%–55%, even after surgical excision of the pathologic tissue [5]. This is an indication that residual disease is present even if it macroscopically seems that the complete lesion has been removed. Postoperative radiotherapy has gained ground over the past few years as a very effective treatment option [9]. Compared to surgery-alone, resection of the PVNS lesion followed by adjuvant radiotherapy of a moderate dose ranged between 30 to 38 Gy, presented lower recurrence rate, increase in the period between the end of treatment and the time of recurrence, the limb function and the radiation-induced toxicity [10-17].
Case ReportA 37-year-old male patient presented with a 6-month history of swelling in the region of the knee joint, accompanied by progressively deteriorating pain and reduced joint functionality. He had a free personal (no trauma) and family medical history. Plain radiographs of the knee joint demonstrated no significant abnormalities and thus it was diagnosed and treated as chondromalacia patellae.
As treatment for chondromalacia patellae showed no improvement of the symptoms, further investigation and new imaging of the joint region was conducted. New plain radiographs presented no significant abnormalities and so magnetic resonance imaging (MRI) was performed (Fig. 1A, 1B). The MRI revealed diffuse tissue development among the soft tissue of the popliteal region, at the posterior site of femur and tibia and along the tendon of the popliteus muscle and—in smaller scale—of the tibiofemoral compartment, the patellofemoral joint and the suprapatellar bursa. The lesion in the popliteal region presented nodular configuration, low signal intensity and an inside part with high signal intensity supporting the diagnosis of PVNS. The pathologically developed tissue appeared to cause relatively small bone erosion of the femoral condyles, both lateral and medial. Also, a small part of the posterior horn (posterior third) of the medial meniscus demonstrated pathologically low intensity signal and chondromalacia lesions in a small part of the femoral articular cartilage. Finally, the MRI revealed suprapatellar joint effusion, as well as effusion of the bursa near the tendon of the semi-membranosus muscle (Fig. 1B). Except for PVNS, differential diagnosis also included synovial chondromatosis, chondroma and synovitis. Laboratory tests’ results showed a borderline low hematocrit value (37.9%; normal laboratory value of 38.3%–48.6%), low hemoglobin value (11.8 g/dL; normal laboratory value of 13.2–16.6 g/dL), low mean corpuscular volume (MCV) value (63.1 fL; normal laboratory value of 80–94 fL), low mean corpuscular hemoglobin (MCH) value (19.6 pg/cell; normal laboratory value of 29 ± 2 pg/cell), low mean corpuscular hemoglobin concentration (MCHC) value (31.1 g/dL; normal laboratory value of 32–36 g/dL), normal platelet count (353,000; normal laboratory value of 150,000–450,000), normal white blood cell count (8,100/mm3; normal laboratory value of 4,500–11,000/mm3) and all the biochemical tests results were normal too.
The definite diagnosis of PVNS was set by the histopathological and immunohistochemical examination of tissue samples taken from the anterior site of the patellofemoral joint as more superficial site of the lesion. The histopathological examination of the biopsy showed synovial membrane characterized by papillary and solid structure, synoviocyte hyperplasia, many hemosiderin granules and intense inflammatory infiltration of lymphocytes, plasmatocytes, a lot of histiocytes/macrophages and a few multinucleated giant cells. The immunohistochemical evaluation was positive for the anti-CD68 antibodies, the histiocytes, the anti-CD45/LCA (leukocyte common antigen) and the lymphocytes, and negative for the anti-S-100, anti-CD34, anti-vimentin, anti-SMA (smooth muscle antibody), anti-Desmin, anti-AEI/AE3 and anti-HMB-45 antibodies. No histological features associated with malignancy had been identified (Fig. 2A, 2B).
The treatment plan included the surgical excision of the PVNS lesion with open synovectomy and then adjuvant radiotherapy. For the total excision of this diffuse PVNS lesion, more than one operation was required. Through the first surgical procedure, the biggest part of the lesion located at the posterior side of the tibiofemoral joint was removed through a posterior knee approach. Taking into account the affection by the lesion to nearby structures such as nerves and blood vessels, a critical intra-operative point, the remnant residual disease was considered to be the minimum possible. A second operation was performed 2 months later, so as to remove the residual mass of the anterior side of the tibiofemoral joint by an anterior incision and medial parapatellar approach. The patient then underwent adjuvant postoperative external radiotherapy four weeks after the second surgical operation in order to reduce the recurrence and improve the local control rates [10-17]. The patient received a regimen of 38 Gy in 19 fractions, at 2.0 Gy/fraction, five times a week, using three-dimensional conformal radiotherapy (3DCRT) technique. The patient underwent computed tomography (CT) simulation in supine position. Definition of the clinical target volume included the entire PVNS mass. The planning target volume was defined by adding a radial margin of only 5 mm in all directions, in order to avoid lower limb radiation-induced lymphoedema. In the current literature, there is a guideline scarcity, regarding the appropriate delineation margins. The modality of image-guided radiation therapy technique was the use of cone-beam CT. In Fig. 3A–3D, we provide the radiotherapy details, such as target volume, dose-volume histogram, as well as radiation treatment planning system information. There was no severe acute toxicity, except for grade 1 dermatitis during and after the whole radiotherapy course. Soon after the first surgery, the patient started physiotherapy sessions, which he received in the following 3 months.
Almost 2 months after the last surgery and a few days after the last radiotherapy fraction, the maximum range of the knee joint moves achieved was -5º of extension to 80º of flexion. Eighteen months after the end of the treatment, the MRI presented no significant alterations. Maximum range achieved was 0º of extension to 90º of flexion. Three years after the end of the treatment, the MRI again demonstrated no pathological alterations (Fig. 4). Maximum range achieved was 0º of extension to 110º of flexion. Neither imaging nor clinical orthopedic evaluation revealed recurrence of the disease. The patient was asymptomatic and had no functional limitations.
DiscussionDiffuse PVNS is a clinical entity that needs careful management in its diagnosis and treatment. The pathological identification of no malignant features is very important. Two or more surgical procedures may be needed for the total or near total excision of the lesion. In cases it affects important nearby structures (nerves and blood vessels) the near total excision is the unavoidable way. The goals of PVNS treatment are the following: (1) preventing disease recurrence, (2) relieving pain and stiffness, and (3) preserving joint function [12]. Postoperative radiotherapy has proved to be a very useful therapeutic option for patients with diffuse PVNS, offering important long-term efficacy and lower recurrence rate [18,19]. In patients with diffuse PVNS of the knee, the delivery of postoperative radiotherapy can achieve: (1) recurrence rate reduction, (2) higher local control rates, (3) good joint function and (4) limited radiation-induced morbidity [12,19-21]. Local control is defined as (1) the absence of disease clinical evidence (including swelling sensation, effusion, and locking of the joint) or (2) stable disease in MRI imaging, for at least 2 years post-treatment [19,20]. Despite the well-recognized disease control advantage of adjuvant radiotherapy, there is no consensus on the optimal dose and regimen at present. The most applied RT regimen was 30–36 Gy in 14–18 fractions [21]. Chien et al. [21] delivered adjuvant radiotherapy with a median dose of 35 Gy (range, 20 to 40 Gy). After median follow-up of 6 years, the recurrence rate was significantly lower in the open synovectomy + postoperative radiotherapy group, than the open synovectomy group (8.3% vs. 57.1%, p = 0.038). The German Cooperative Group on Radiotherapy for Benign Diseases conducted a multicenter pattern of care study regarding radiotherapy for PVNS, presenting data for 41 patients from 14 radiotherapy departments. Patients received a median dose of 36 Gy (range, 30.0 to 50.4 Gy) with the median single dose being 2.0 Gy (range, 1.8 to 2.5 Gy) in 5 fractions per week. In-field recurrence occurred in two patients (4.9%) both of whom received a total dose of 36 Gy in single doses of 1.8 Gy and 2.0 Gy, respectively. Moderate or serious functional impairment was observed in five patients (5/39; 12.2%) and crude outcomes of radiotherapy were of no higher significance than that of Radiation Oncology Treatment Group grade II. Temporary skin erythema (grade I) was observed in 11 cases, skin fibrosis and hyperpigmentation in four patients and lymphedema in one case [22]. Although classified as a benign disease, diffuse PVNS is more destructive and may cause severe disability. Despite having a high recurrence rate, there is a lack of strong evidence regarding the optimal diffuse PVNS management. Postsurgery radiotherapy should be considered, due to the achievement of higher local rates, with minimal acute or late radiation-induced toxicity, using 3DCRT or intensity-modulated radiation therapy techniques [4,12,16-22].
Fig. 1.(A) Plain anterior-posterior radiograph of the knee. No significant abnormalities. (B) Sagittal view of the left knee with three-dimensional fast spoiled gradient-echo (FSPGR) sequence. Extensive diffuse pigmented villonodular synovitis occupying the posterior and anterior part of the knee joint with extra-articular extension demonstrated with low to medium signal and blooming artifact due to hemosiderin (white arrows). 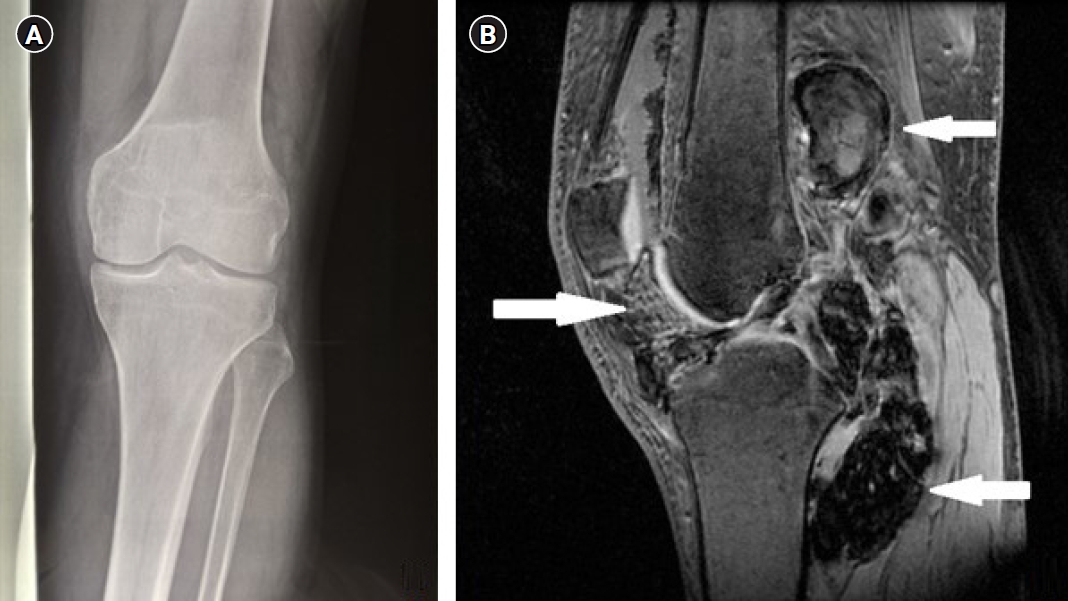
Fig. 2.Diffuse pigmented villonodular synovitis. (A) Histological examination of tissue samples taken from the lesion (H&E staining, original magnification ×20). Synoviocyte hyperplasia, a lot of hemosiderin granules and intense inflammatory infiltration of lymphocytes, plasmatocytes, many histiocytes/macrophages and a few multinucleated giant cells. (B) Immunohistochemical staining of tissue samples taken from the lesion with the anti-CD68 antibody (original magnification ×20). CD68-positive mononuclear cells and multinucleated giant cells detected—CD68 stain in the cytoplasm of those cells. 
References1. Staals EL, Ferrari S, Donati DM, Palmerini E. Diffuse-type tenosynovial giant cell tumour: current treatment concepts and future perspectives. Eur J Cancer 2016;63:34–40.
2. Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol 1941;31:731–65.
3. Byers PD, Cotton RE, Deacon OW, et al. The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Br 1968;50:290–305.
4. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine (Baltimore) 1980;59:223–38.
5. Burton TM, Ye X, Parker ED, Bancroft T, Healey J. Burden of illness associated with tenosynovial giant cell tumors. Clin Ther 2018;40:593–602.
6. Granowitz SP, D’Antonio J, Mankin HL. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop Relat Res 1976;(114):335–51.
7. Hantes ME, Basdekis GK, Zibis AH, Karantanas AH, Malizos KN. Localized pigmented villonodular synovitis in the anteromedial compartment of the knee associated with cartilage lesions of the medial femoral condyle: report of a case and review of the literature. Knee Surg Sports Traumatol Arthrosc 2005;13:209–12.
8. Auregan JC, Bohu Y, Lefevre N, et al. Primary arthroscopic synovectomy for pigmented villo-nodular synovitis of the knee: recurrence rate and functional outcomes after a mean follow-up of seven years. Orthop Traumatol Surg Res 2013;99:937–43.
9. Bruns J, Ewerbeck V, Dominkus M, et al. Pigmented villo-nodular synovitis and giant-cell tumor of tendon sheaths: a binational retrospective study. Arch Orthop Trauma Surg 2013;133:1047–53.
10. Duan Y, Qian J, Chen K, Zhang Z. Necessity of adjuvant postoperative radiotherapy for diffuse pigmented villonodular synovitis of the knee: a case report and literature review. Medicine (Baltimore) 2018;97:e9637.
11. de Carvalho LH, Soares LF, Goncalves MB, Temponi EF, de Melo Silva O. Long-term success in the treatment of diffuse pigmented villonodular synovitis of the knee with subtotal synovectomy and radiotherapy. Arthroscopy 2012;28:1271–4.
12. Griffin AM, Ferguson PC, Catton CN, et al. Long-term outcome of the treatment of high-risk tenosynovial giant cell tumor/pigmented villonodular synovitis with radiotherapy and surgery. Cancer 2012;118:4901–9.
13. Kramer DE, Frassica FJ, Frassica DA, Cosgarea AJ. Pigmented villonodular synovitis of the knee: diagnosis and treatment. J Knee Surg 2009;22:243–54.
14. Nassar WA, Bassiony AA, Elghazaly HA. Treatment of diffuse pigmented villonodular synovitis of the knee with combined surgical and radiosynovectomy. HSS J 2009;5:19–23.
15. Heyd R, Seegenschmiedt MH, Micke O. The role of external beam radiation therapy in the adjuvant treatment of pigmented villonodular synovitis. Z Orthop Unfall 2011;149:677–82.
16. Park G, Kim YS, Kim JH, et al. Low-dose external beam radiotherapy as a postoperative treatment for patients with diffuse pigmented villonodular synovitis of the knee: 4 recurrences in 23 patients followed for mean 9 years. Acta Orthop 2012;83:256–60.
17. Li W, Sun X, Lin J, Ji W, Ruan D. Arthroscopic synovectomy and postoperative assisted radiotherapy for treating diffuse pigmented villonodular synovitis of the knee: an observational retrospective study. Pak J Med Sci 2015;31:956–60.
18. Chen K, Ren Q, Han XR, Zhang XN, Wei B, Bai XZ. Imatinib mesylate induces mitochondria-dependent apoptosis and inhibits invasion of human pigmented villonodular synovitis fibroblast-like synovial cells. Oncol Rep 2016;35:197–204.
19. Berger B, Ganswindt U, Bamberg M, Hehr T. External beam radiotherapy as postoperative treatment of diffuse pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys 2007;67:1130–4.
20. Horoschak M, Tran PT, Bachireddy P, et al. External beam radiation therapy enhances local control in pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys 2009;75:183–7.
|
|
|||||||||||||||||||||||||||||||||||||||||||||

 |
 |