Journey to hypofractionation in radiotherapy for breast cancer: critical reviews for recent updates
Article information
Abstract
Historical conventional fractionated radiation therapy (RT) for breast cancer consisted of 1.8–2.0 Gy per fraction with a total dose of 45–60 Gy over 5–7 weeks. Based on radiobiological characteristics, a low α/β is suspected of breast cancer resulting in sensitivity to higher dose per fraction (2.5–3.0 Gy). Over the past 10 years, multiple clinical trials support the application of shorter treatment regimen with hypofractionated RT (HypoRT). Recently, ultra-HypoRT with 5 fractions showed favorable outcomes. Although the safety and efficacy of HypoRT has been supported by high-quality randomized trials, there are still some worries and doubts around HypoRT from radiation oncologists. However, the radiation oncology community have now reached an important timepoint for adopting HypoRT during the COVID-19 pandemic. The aim of this review is to provide an overview of HypoRT in breast cancer based on prospective randomized trials and discuss the special consideration regarding HypoRT.
Introduction
A historical regimen of 25–28 fractions over 6 weeks was adopted for radiotherapy (RT) following breast-conserving surgery (BCS) and total mastectomy. An early assumption that breast cancer cell lines might be more sensitive to fractional doses than acute skin reactions and other squamous carcinomas led to development of the hypofractionated RT (HypoRT) approach, which elevated fractional dose up to 3 Gy with reduced total dose/fractions, for obtaining radiobiological equivalence to a traditional regimen of 50–50.4 Gy in 25–28 fractions [1,2].
During the last two decades, numerous clinical trials have evaluated shortened courses of RT in the setting of whole breast irradiation (WBI) and chest wall RT. Benefits of HypoRT include both, radiobiologic advantages and reduced length of treatment courses, for improving healthcare resources and patient convenience.
Consequently, HypoRT has been widely adopted worldwide based on a series of randomized clinical trials [3,4]. Recently, HypoRT over 3 weeks and with shorter courses of 5 fractions have been recommended in treatment guidelines [5,6]. Herein, we review the history of clinical trials, summarize current guidelines, raise several issues regarding HypoRT for special conditions, and discuss the future prospects.
Randomized Trials
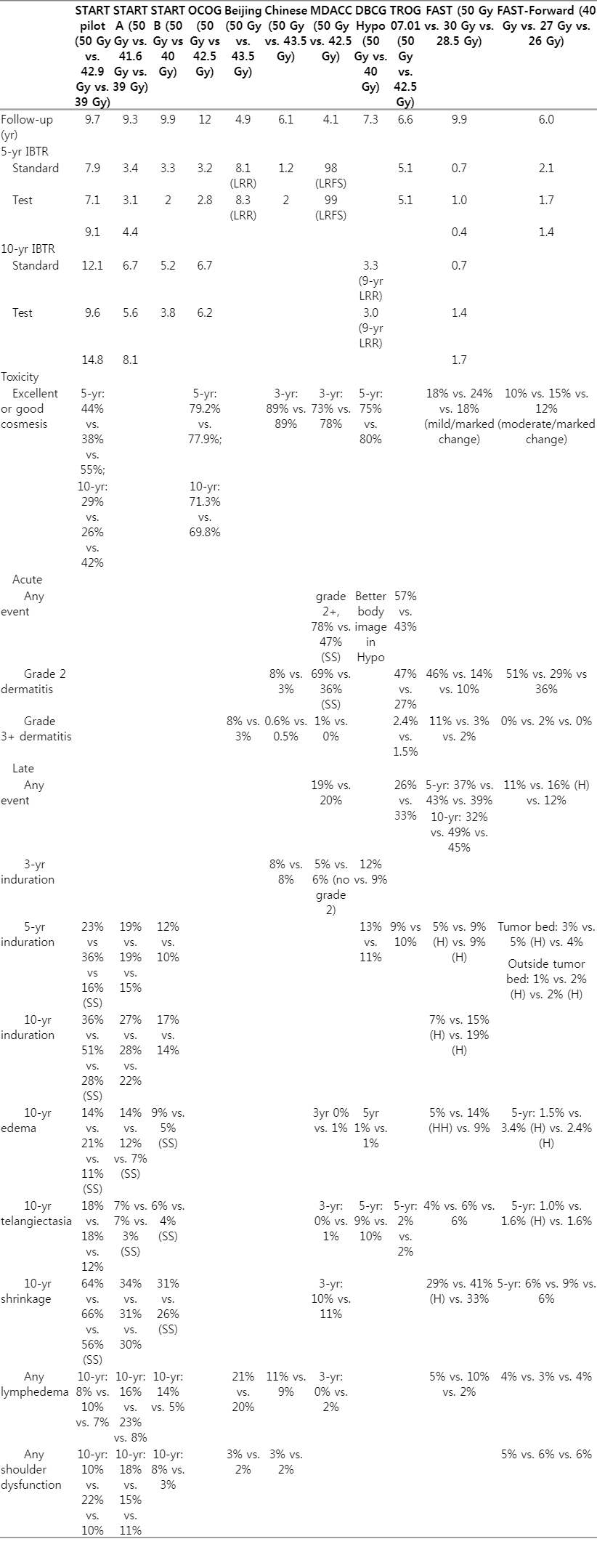
In this review, 11 randomized trials were reviewed for HypoRT in breast cancer patients (Figs. 1, 2). Baseline characteristics and treatment outcomes are summarized in Tables 1 and 2.
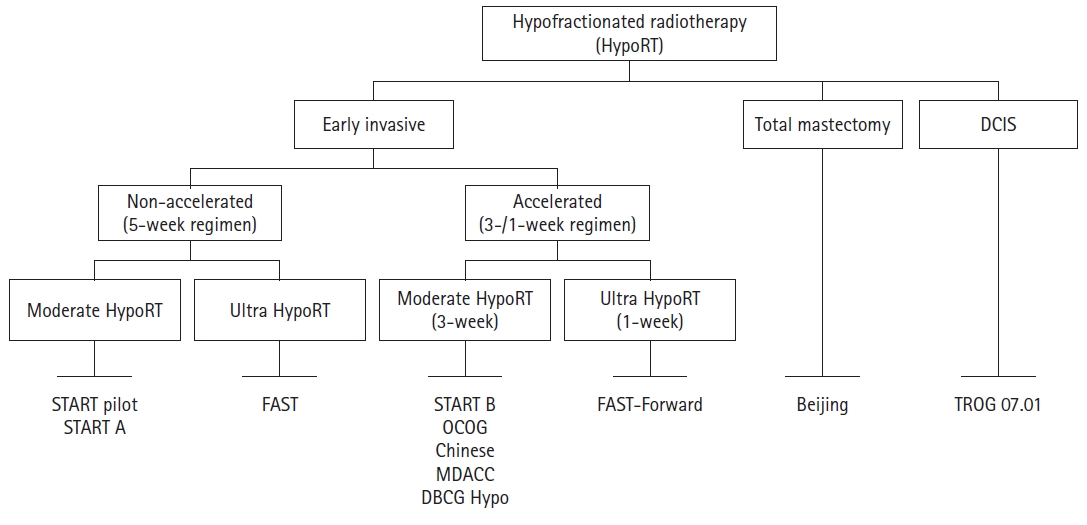
Schema of trials for hypofractionated radiation therapy (Hypo-RT). DCIS, ductal carcinoma in situ; START, Standardization of Breast Radiotherapy Trial; OCOG, Ontario Clinical Oncology Group; DBCG, Danish Breast Cancer Group; MDACC, MD Anderson Cancer Center; TROG, Trans-Tasman Radiation Oncology Group.
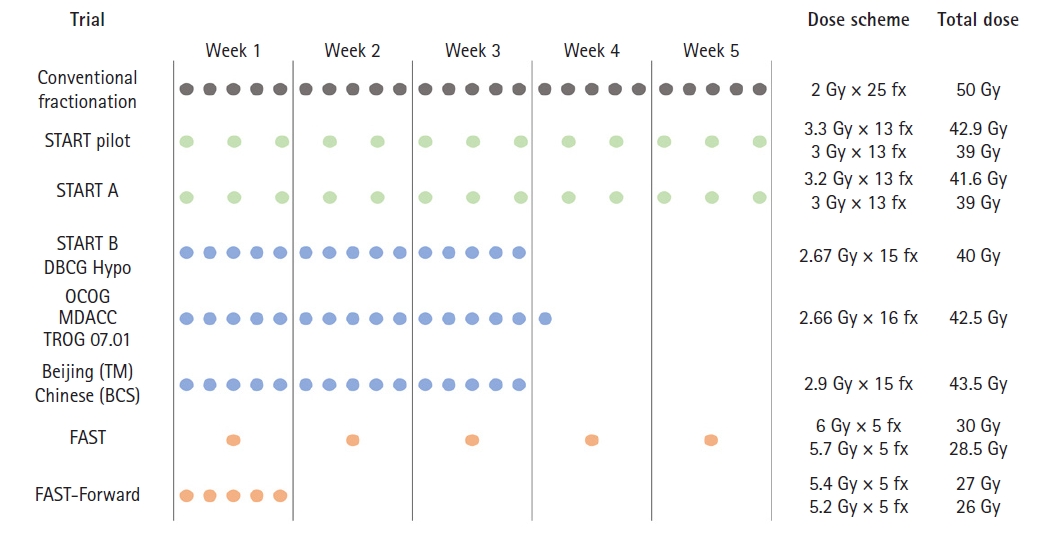
Treatment schedules and dose regimens of trials for hypofractionated radiation therapy. BCS, breast-conserving surgery; TM, total mastectomy; START, Standardization of Breast Radiotherapy Trial; OCOG, Ontario Clinical Oncology Group; DBCG, Danish Breast Cancer Group; MDACC, MD Anderson Cancer Center; TROG, Trans-Tasman Radiation Oncology Group.
1. Standardization of Breast Radiotherapy (START) pilot
The Royal Marsden Hospital/Gloucestershire Oncology Center enrolled 1410 pT1-3N0-1 breast cancer patients who underwent BCS between 1986 and 1998. All patients were treated over 5 weeks; a conventional fractionated regimen of 50 Gy/25 fx was compared with HypoRT of 42.9 Gy or 39 Gy/13 fx. This trial was controlled for overall treatment time and was designed to estimate α/β for late changes in breast appearance. The risk of any change in breast appearance after at least 5 years of 50 Gy/25 fx, 42.9 Gy/13 fx, and 39 Gy/13 fx was 39.6%, 45.7%, and 30.3%, respectively. An α/β value of 3.6 Gy was generated for change in photographic breast appearance [7]. Moreover, 39 Gy/13 fx was related to reduced breast induration, edema, and breast shrinkage. The secondary endpoint of ipsilateral breast tumor recurrence (IBTR) was comparable among treatment groups; the 10-year IBTR rates after 50 Gy/25 fx, 42.9 Gy/13 fx, and 39 Gy/13 fx were 12.1%, 9.6%, and 14.8%, respectively. They also generated an α/β value of 4.0 Gy for tumor control, similar to that for change in photographic breast appearance [8].
2. START-A
A 5-week HypoRT regimen was also evaluated in START-A trial. Between 1998 and 2002, 2,236 patients with pT1-2 were enrolled to test IBTR as the primary endpoint, following the same design as START-pilot study. The 10-year IBTR rate was 6.7%, 5.6%, and 8.1% for 50 Gy/25 fx, 41.6 Gy/13 fx, and 39 Gy/13 fx arms, respectively [9]. In addition, late normal tissue effects, including induration, telangiectasia, and edema, were significantly reduced in 39 Gy/13 fx arm compared with 50 Gy/25 fx arm. Nevertheless, there was no significant difference in normal tissue effect between 50 Gy/25 fx and 41.6 Gy/13 fx arm; the estimated α/β value for any change in breast appearance was 3.1 Gy. Furthermore, in a combined meta-analysis with START-pilot trial, the α/β value for IBTR was 3.5 Gy.
3. Ontario Clinical Oncology Group (OCOG), START-B
Both OCOG and START-B trial compared HypoRT over 3 weeks, with 50 Gy/25 fx over 5 weeks.
The OCOG trial randomized 1,234 pT1-2N0 breast cancer patients following BCS to 50 Gy/25 fx or 3-week regimen of 42.56 Gy/16 fx [10]. The primary endpoint of IBTR was comparable between the two groups; 10-year IBTR rates were 6.7% and 6.2% in 50 Gy/25 fx and 42.56 Gy/16 fx arms, respectively. Additionally, global cosmetic outcomes indicated a comparable rate of excellent/good cosmesis at 10 years after 50 Gy (71.3%) and 42.56 Gy (69.8%). Moreover, the two groups did not differ in late toxic effects on the skin or subcutaneous area.
The START-B trial evaluated the 3-week HypoRT regimen of 40 Gy/15 fx compared with 50 Gy/25 fx over 5 weeks in 2,215 pT1-2N0-1 breast cancer patients [11]. At 10 years, rates of IBTR did not differ significantly between 50 Gy/25 fx (5.5%) and 40 Gy/15 fx (4.3%) arms [9]. Long-term follow-up data revealed favorable late normal tissue effects such as moderate/marked shrinkage, telangiectasia, and breast edema after 40 Gy/15 fx rather than after 50 Gy/25 fx.
In addition, a post-hoc analysis for START-pilot, START-A, and START-B demonstrated that treatment effect (HypoRT vs. 50 Gy/25 fx) was similar in both IBTR and moderate/marked normal tissue effects irrespective of various patient and tumor characteristics [9]. Furthermore, late adverse events of cardiac toxicity, rib fracture, and lung fibrosis were rare after both, HypoRT and 50 Gy/25 fx arm in both START-A and START-B trials.
In the modern era, studies from the Danish Breast Cancer Group (DBCG), MD Anderson Cancer Center (MDACC), Beijing, Chinese trials, and Breast International Group/Trans-Tasman Radiation Oncology Group (TROG) report outcomes were consistent with the earlier trials.
4. DBCG HYPO
The DBCG-HYPO trial (2009–2014; n=1,854) investigated the occurrence of breast induration following the 3-week regimen of 40 Gy/15 fx compared with 50 Gy/25 fx [12]. They also included 13% of ductal carcinoma in-situ (DCIS). At the 3-year follow-up, induration was observed similarly in both groups (12% vs. 9% in 50 Gy/25 fx and 40 Gy/15 fx, respectively; p = 0.070), which satisfied the predetermined non-inferiority margin for 40 Gy/15 fx arm. In addition, the rate of overall cosmetic outcome (excellent/good cosmesis) was comparable between the two groups (75% vs. 80% in 50 Gy/25 fx and 40 Gy/15 fx, respectively). In both DCIS and invasive cancer subgroups, 40 Gy/15 fx and 50 Gy/25 fx had no difference in locoregional recurrence rate.
5. MDACC
From 2011 to 2014, MDACC enrolled 287 patients with stage 0–II breast cancer (DCIS of 22%, invasive cancer of 78%) for randomization to 50 Gy/25 fx and 3-week regimen of 42.6 Gy/16 fx arm [13,14]. All patients underwent BCS and received a tumor bed boost. Physician-assessed excellent/good cosmetic outcomes showed no difference between treatment groups (73% vs. 78% in 50 Gy/25 fx and 42.4 Gy/16 fx, respectively). In addition, they showed an excellent 9-year local recurrence-free survival rate of 98% and 99%, respectively. The grade 2 or more acute toxicities rate was less with 42.6 Gy/16 fx than with 50 Gy/25 fx (47% vs. 78%, p < 0.01). Specifically, acute dermatitis, pruritis, breast pain, and hyperpigmentation were significantly less observed in 42.6 Gy/16 fx than 50 Gy/25 fx arm. There was no difference in the late adverse event of induration, edema, telangiectasia, shrinkage, or lymphedema.
6. Beijing
Regarding post-mastectomy radiation therapy, in Beijing, China, 820 patients with locally advanced breast cancer (pT3-4 or pN2, stage-III 94%) were randomized to 50 Gy/25 fx or 43.5 Gy/15 fx (3-week) between 2008 and 2016 [15]. All patients received systemic chemotherapy, wherein 25% and 75% received neoadjuvant and adjuvant chemotherapy, respectively. At a median follow-up of 4.9 years, the 5-year locoregional recurrence rates were 8.1% and 8.3% for 50 Gy/25 fx and 43.5 Gy/15 fx arm, respectively, satisfying a predefined non-inferiority margin. Furthermore, acute and late toxicities were comparable between the two groups, except that HypoRT of 43.5 Gy/15 fx had less grade 3 acute skin toxicity than the 50 Gy/25 fx arm (3% vs. 8%; p < 0.001). None of the patients experienced brachial plexopathy, and grade 3 lymphedema was infrequently observed (<1% in both groups).
7. Chinese
Recently, an Asian multi-institutional trial included 734 patients with pT1-2 disease following BCS between 2010 and 2015. The patients were assigned to 50 Gy/25 fx followed by 10 Gy/5 fx boost or 3-week HypoRT of 43.5 Gy/15 fx followed by 8.7 Gy/3 fx boost [16]. In this trial, most patients received a sequential tumor bed boost (99.7%) and an intensity-modulated RT (IMRT) technique (either forward or inverse planning, 97.4%). With a median follow-up of 6.1 years, there was no significant difference in 5-year IBTR rate of 1.2% and 2.0% in the 43.5 Gy/15 fx and 50 Gy/25 fx arms, respectively. With regard to toxicities, acute skin toxicity of grade 2/3 was frequently observed after 50 Gy/25 fx than 43.5 Gy/15 fx (8% vs. 3%; p = 0.019). Overall cosmetic outcomes were comparable between the two groups: both 89% of patients experienced excellent/good cosmesis after 50 Gy/25 fx and 43.5 Gy/15 fx, respectively (p = 0.393).
8. TROG 07.01
Although previous trials included patients with DCIS, their number was limited to perform a meaningful statistical analysis. In this regard, a recent international multicenter randomized trial (TROG 07.01) evaluated 3-week HypoRT (42.5 Gy/16 fx) compared with 50 Gy/25 fx in patients with non-low-risk DCIS [17]. There were 1,147 patients with increased risk of IBTR, including young (<50 years) or old (≥50 years) patients with the presence of one or more of the following factors: symptomatic presentation, size ≥15 mm, multifocal disease, intermediate/high grade, central necrosis, comedo histology, and margin <10 mm. With a median follow-up of 6.6 years, 5-year freedom from IBTR rates were 94.9% and 94.9% following 42.5 Gy/16 fx and 50 Gy/25 fx, respectively. The crude rates of grade 2 or more acute radiation dermatitis were 27% and 47% and those of late breast induration at 5 years were 10% and 9% following 42.5 Gy/16 fx and 50 Gy/25 fx, respectively. Additionally, none of the categories of patient-reported outcomes except for body image and sexuality differed significantly between 42.5 Gy/16 fx and 50 Gy/25 fx arms [18]. Patients treated with 42.5 Gy/16 fx showed better body image and sexuality than those treated with 50 Gy/25 fx.
Recently, FAST Trial Management Group investigated a substantial step forward in ultra-hypofractionated (ultra-HypoRT) regimens.
9. FAST
Once-a-week ultra-HypoRT was investigated in the UK-FAST (CRUKE/04/015) trial conducted on 915 patients from 2004 to 2007 [19]. Patients aged ≥50 years and those with pT1-2N0 disease were assigned for 50 Gy/25 fx and 30 or 28.5 Gy/5 fx (once-a -week). The primary endpoint was a change in photographic breast appearance at 2 and 5 years. In terms of acute radiation dermatitis, patients in both 30 Gy/5 fx and 28.5 Gy/5 fx arms showed less common ≥grade 3 dermatitis than those in 50 Gy/25 fx arm (30 Gy, 2.7%; 28.5 Gy, 1.9%; and 50 Gy, 10.9%). Mild and marked changes through photographic assessment at 5 years were significantly more common after 30 Gy/5 fx (24%) compared with 50 Gy/25 fx (18%) and 28.5 Gy/5 fx (18%). Applying this result, an estimation of α/β value for photographic endpoint was 2.7 Gy, iso-effective of 28.5 Gy/5 fx regimen compared to 50 Gy/25 fx. With a follow-up of 9.9 years, a total of 11 IBTR events were observed; 10-year estimated IBTR rates were 0.7%, 1.4%, and 1.7% in the 50 Gy/25 fx, 30 Gy/5 fx, and 28.5 Gy/5 fx arms, respectively.
10. FAST-Forward
The FAST-Forward investigated non-inferiority of a very accelerated course of HypoRT (27 or 26 Gy/5 fx) over 1 week compared with a 3-week moderately paced course of HypoRT 40 Gy/15 fx [20]. A total of 4,096 patients with pT1-3N0-1 disease following BCS or mastectomy were enrolled. Most patients had pT1 (90%) and pN0 (82%) disease, and estrogen receptor positive tumor (80%). At a median follow-up of 6.0 years, 5-year cumulative incidence of IBTR rates (primary endpoint) was comparable among treatment groups: 2.1%, 1.7%, and 1.4% after 40 Gy/15 fx, 27 Gy/5 fx, and 26 Gy/5 fx, respectively, which satisfied the non-inferiority margin. Regarding late normal tissue effects, 27 Gy/5 fx arm displayed a significantly higher risk of late normal tissue effect, whereas 26 Gy/5 fx showed results comparable to 40 Gy/15 fx. However, moderate/marked breast induration outside the tumor bed was frequently observed in both 26 Gy/5 fx (1.6%) and 27 Gy/5 fx (2.3%) arms compared with 40 Gy/15 fx (0.8%) arm.
Current Guidelines
Based on the aforementioned high-quality randomized trials, the American Society for Radiation Oncology (ASTRO) has endorsed HypoRT in WBI regardless of age, stage, and administration of chemotherapy [6]. The National Comprehensive Cancer Network (version 4, 2022) also suggests HypoRT only for WBI; a FAST regimen of 28.5 Gy/5 fx (over 5 weeks) could be considered for age >50 years with pT1-2N0 disease. However, a recent consensus recommendation from the European Society for Radiotherapy and Oncology supports comprehensive implementation of HypoRT (15–16 fx) [5]. They state that HypoRT can be adopted for patients treated with whole breast, chest wall (regardless of reconstruction), and regional node irradiation (RNI). Moreover, consensus was reached for offering ultra-HypoRT (5 fx in 1 week) as either standard of care or trial/registry-based care in WBI.
Special Considerations
1. DCIS
As mentioned above, the efficacy of HypoRT in DCIS remains questionable as previous studies of START and OCOG trials did not include patients with DCIS. A recent randomized study from MDACC only included 22% patients with DCIS [13,14]. However, the recent TROG 07.01 trial demonstrated that HypoRT does not compromise local control outcomes compared to conventional fractionated RT, as in invasive cancer [17].
2. Regional node irradiation
Although RNI was scarcely represented in START trials (15%), there was no significant difference in long-term patient/physician-assessed arm or shoulder symptoms [21]. Also, a Beijing trial of postmastectomy HypoRT (43.5 Gy/15 fx vs. 50 Gy/25 fx) with RNI reported equivalent late normal tissue events [15]. Badiyan et al. [22] reviewed published literature evaluating late toxicities, including cardiotoxicity, pneumonitis, lymphedema, and brachial plexopathy. They concluded that HypoRT did not jeopardize late adverse events, warranting a good safety profile of HypoRT with RNI. Upcoming results of UK IMPORT High (NCT00818051) and DBCG SKAGEN Trial 1 (NCT02384733) are expected to clarify the safety issue of HypoRT in RNI.
3. Reconstruction
To date, there are limited data for HypoRT in the reconstruction setting. Previous small series have reported the importance of total dose in major reconstruction-related complications (e.g., capsular contracture, implant failure) [23,24]. Despite no randomized trial, HypoRT has been adopted for all types of breast reconstruction in many countries, especially around Europe [4]. Ongoing trials of FABREC (NCT03422003) and Alliance A221505 (RT CHARM, NCT03414970) will provide evidence of HypoRT use in the reconstruction setting.
4. Boost
Tumor bed boost is recommended to reduce IBTR events following BCS for invasive cancer or DCIS [17,25]. Apart from >2 Gy per fraction for WBI, boost irradiation was administered sequentially with 10–16 Gy in 5–8 fractions in previous trials (START, DBCG-HYPO, and TROG 07.01) [7,9,11,12]. Chinese trial (43.5 Gy/15 fx vs. 50 Gy/25 fx) adopted boost irradiation of 8.7 Gy/3 fx in HypoRT arm [16]. Acute and late toxicities were similar between 43.5 Gy/15 fx followed by 8.7 Gy/3 fx and 50 Gy/25 fx followed by 10 Gy/5 fx, except for acute radiation dermatitis favoring HypoRT arm. A boost of 2.5 Gy per fraction was administered for HypoRT (42.6 Gy/16 fx) in a randomized trial at MDACC [13,14]. It demonstrated that 2.5 Gy per fraction boost irradiation was not a strong contraindication in the setting of HypoRT.
The shift towards reducing overall treatment time has led to a simultaneous integrated boost for HypoRT. Based on several phase I-II prospective trials [26-29], phase III randomized trials—Radiation Therapy Oncology Group 1005 (NCT01349322), Hypofractionation with simultaneous integrated boost (HYPOSIB; NCT02474641), and UK IMPORT High (NCT00818051)—comparing simultaneous integrated and sequential (12–16 Gy/6–8 fx) boosts were conducted to clarify the safety and efficacy of simultaneous boost with increased fractional dose along with HypoRT in WBI. Preliminary results from HYPOSIB showed that acute skin reactions after a simultaneous boost of 48 Gy/15 fx were less pronounced and reached an earlier peak than those after the control arm [30].
5. IMRT
Despite technical advances in RT planning for breast cancer, techniques used in previous randomized trials are slightly outdated. Previous trials mostly adopted two-dimensional RT, while recent trials have adopted CT-based three-dimensional conformal RT. Although the Beijing trial from China stated that it used IMRT in 97.4% cases, both forward and inverse planning IMRT existed [16]. Given the sizeable fractional dose in HypoRT, an increased point dose ("hot spot") could induce unexpected adverse events. Since controlling unplanned dose inhomogeneities in forward planning or conventional wedge is quite challenging, inverse planning IMRT could improve dose conformity, satisfying dose-volume restriction for normal organs [31]. IMRT can enable the safe simultaneous boost in HypoRT. The simultaneous boost trials, mentioned above, mostly adopted IMRT techniques.
A large retrospective data from 5,749 patients demonstrated that HypoRT with IMRT reduced grade 2 or more acute/subacute toxicities compared with HypoRT with conventional techniques and conventional fractionated RT [32]. They also demonstrated that the benefit of HypoRT with IMRT is maximized in patients treated with RNI.
Although concerns around increased risk of secondary malignancy from increased dose to the contralateral breast exist, comparative analyses from National Cancer Database and large retrospective data have revealed a comparable risk of secondary cancer after IMRT compared to conventional techniques [32,33].
Based on this recent evidence, the ASTRO has withdrawn the previous Choosing Wisely recommendation announced in 2013, which hindered routine use of IMRT to WBI.
Conclusion
Given excellent IBTR control rates and toxicity profiles from existing data, HypoRT is an efficient, safe, and convenient treatment approach for breast cancer. The standard of care for adjuvant RT has shifted from 5–6 weeks of conventional fractionated RT to 3–4 weeks of HypoRT. Newer trials conducted with 5 fractions might further change the standard of care with long-term follow-up data. In the years to come, breast radiation is expected to further evolve based on radiobiological consideration, allowing for shorter regimens.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author Contributions
Conceptualization, YBK; Investigation and methodology, NK, YBK; Project administration, YBK; Resources, NK, YBK; Supervision, YBK; Writing of the original draft, NK; Writing of the review and editing, NK, YBK; Software, NK; Visualization, NK. All authors have proofread the final version.