The effects of low-dose radiation therapy in patients with mild-to-moderate Alzheimer's dementia: an interim analysis of a pilot study
Article information
Abstract
Purpose
We aimed to determine whether low-dose radiotherapy (LDRT) is effective in patients with Alzheimer disease (AD).
Materials and Methods
We included patients according to the following criteria: probable Alzheimer's dementia according to the New Diagnostic Criteria for Alzheimer’s Disease; confirmation of amyloid plaque deposits on baseline amyloid positron emission tomography (PET); a Korean Mini-Mental State Examination 2nd edition (K-MMSE-2) score of 13–26; and a Global Clinical Dementia Rating (CDR) score of 0.5–2 points. LDRT was performed six times at 0.5 Gy each. Post-treatment cognitive function tests and PET-CT examinations were performed to evaluate efficacy. The medication for AD treatment was maintained throughout the study period.
Results
At 6 months after LDRT, neurological improvement was seen in 20% of patients. Patient #2 showed improvement in all domains of the Seoul Neuropsychological Screening Battery II (SNSB-II). Moreover, the K-MMSE-2 and Geriatric Depression Score-Short Form scores improved from 20 to 23 and from 8 to 2, respectively. For patient #3, the CDR score (sum of box score) improved from 1 (4.0) to 1 (3.5) at 3 months follow-up. Moreover, the Z scores for language and related functions, memory, and frontal executive function improved to -2.56, -1.86, and -1.32, respectively at the 6-month follow-up. Two patients complained of mild nausea and mild hair loss during LDRT, which improved after treatment.
Conclusion
One of the five patients with AD treated with LDRT experienced a temporary improvement in SNSB-II. LDRT is tolerable in patients with AD. We are currently under follow-up and will conduct cognitive function tests after 12 months after LDRT. A large-scale randomized controlled trial with a longer follow-up period is warranted to determine the effect of LDRT on patients with AD.
Introduction
Alzheimer’s disease (AD) is the most prevalent causative disease for dementia [1]. In 2017, there were approximately 50 million people with dementia worldwide, with this number being expected to double every 20 years [1]. AD is a neurodegenerative disease involving complex interactions among amyloid-β, tau, and neuroinflammation. Although clinical trials have investigated the efficacy of drugs targeting amyloid-β and tau, there remains no effective treatment [2,3]. Acetylcholinesterase inhibitors, including donepezil, rivastigmine, galantamine, and tacrine, as well as the N-methyl-D-aspartate (NMDA) antagonist memantine, have been approved by the US Food and Drug Administration; however, they only provide temporary symptom relief [4]. There has been increasing research on neuroinflammation in AD, which is characterized by the presence of reactive astrocytes and activated microglial cells mainly around amyloid plaques. Neuroinflammation is crucially involved in the pathophysiology of AD [5]. Natural substances with anti-inflammatory effects have been investigated in studies on AD treatment; however, they have limited ability to cross the blood-brain barrier or are rapidly metabolized and eliminated [6]. Recent studies have demonstrated the anti-inflammatory effects of low-dose radiotherapy (LDRT) [7-9], which has shown efficacy against neuroinflammation in AD. Specifically, relatively low-to-moderate total doses of 9–10 Gy in 5 fractions have been shown to significantly reduce the amyloid plaque burden and/or tau staining in AD mouse models [10-13]. These previous studies applied a relatively low total dose of 10 Gy, which is lower than the 60 Gy generally used in cancer treatment; however, the dose per fraction remained similar to the conventional dose RT (1.8–2 Gy). Another research group has attempted treating AD using much lower RT doses. Yang et al. [14] revealed a proper RT dose and schedule for late-stage AD using 8- and 9-month-old 5xFAD mice, which is an established animal model for AD, by comparing the low total dose with a low single dose (5 × 0.6 Gy) and the low moderate total dose with conventional dose (5 × 2 Gy). They found that LDRT involving 5 fractions at 3 Gy could effectively reduce beta-amyloid in the brains of mice with AD and improve cognitive function. Further, the anti-inflammatory effect was confirmed by changes in cytokine levels. Therefore, we aimed to determine whether LDRT was effective in patients with AD.
Materials and Methods
1. Patients
The inclusion criteria were as follows: (1) men and women aged 60–85 years; (2) probable Alzheimer's dementia according to the New Diagnostic Criteria for Alzheimer’s Disease (NIA-AA) [15]; (3) confirmation of amyloid plaque deposits on baseline amyloid positron emission tomography (PET); (4) mild-to-moderate AD with a Korean Mini-Mental State Examination 2nd edition (K-MMSE-2) score of 13–26; (5) Global Clinical Dementia Rating (CDR) score of 0.5–2 points; (6) ability to perform cognitive and other tests; (7) having an accompanying guardian (a family member or a trusted person who takes care of daily life) during the visits; (8) having provided written informed consent; and (9) having maintained the existing drug treatment for general dementia within >3 months.
The exclusion criteria were as follows: (1) possible or probable vascular dementia according to the National Institute of Neurological Disorders and Stroke (NINDS) and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (AIREN) criteria [16]; (2) a history of other central nervous diseases as the main cause of dementia (cerebrovascular disease; structural or developmental malformation; epilepsy; infectious, degenerative, or infectious/demyelinating history) or presence of the corresponding evidence on brain MRI scans performed within the past 12 months or at screening; (3) uneducated or illiterate; (4) severe hearing and vision impairment that impeded efficacy evaluation; (5) abnormal test results for vitamin B12, syphilis serology, or thyroid stimulating hormone that may worsen or cause dementia; (6) a history of psychiatric diseases that may impede trial participation, including schizophrenia or bipolar disorder, or currently having severe depression (Geriatric Depression Score-Short Form [SGDS] ≥12); (7) a diagnosis of a malignant tumor within 5 years from the screening time; or (8) a history of brain irradiation for therapeutic intent.
2. Study design
After approval from the Institutional Review Board of Chungbuk National University Hospital (IRB No. 2021-04-011-007), this prospective, single-center, investigator-initiated pilot study was conducted to evaluate the safety and effectiveness of LDRT for AD. The study period was from June 2021 to June 2022, and a total of seven subjects were planned to be recruited. The patients underwent whole-brain irradiation involving six doses of LDRT (0.5 Gy). After the end of treatment, the patients underwent cognitive function tests and PET-CT follow-ups for efficacy evaluation (Fig. 1). The medication for AD treatment was maintained. Beyond 6 months follow-up, further visit will be continued through an outpatient of neurology department.
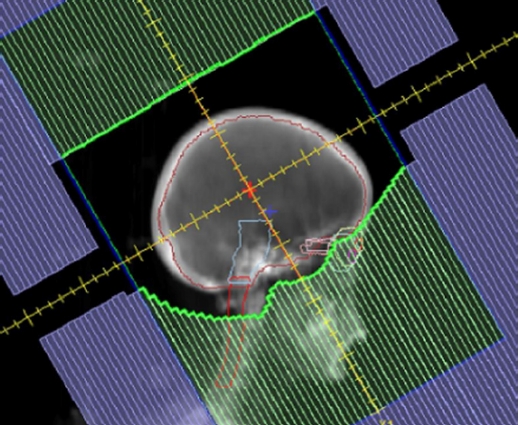
3. Radiotherapy
The patients underwent treatment planning computed tomography (CT) while immobilized. The clinical target volume (CTV) was measured within the whole brain. The planning target volume (PTV) was determined by expanding the CTV by 5 mm. The inferior border of the field was placed at the inferior endplate of the C1 vertebra (Fig. 2). The prescribed dose of the PTV for whole-brain RT was 3 Gy, 0.5 Gy/time, and three times/week. Regarding the planned optimization goals, >95% of the PTV received the prescribed dose. A linear accelerator (LINAC) Versa HD (ELEKTA, Stockholm, Sweden) with low-dose-rate photons (6 MV, 100 MU/min) was used for treatment delivery.
4. Outcome measures
Neurocognitive function was evaluated using the Seoul Neuropsychological Screening Battery II (SNSB-II) at baseline as well as 3 and 6 months after the LDRT schedule. The primary outcome was the change in the CDR-sum of boxes (SB) score. The secondary outcomes were the change in the K-MMSE-2, z-score of each SNSB domain, Korean Instrumental Activities of Daily Living Scale (K-IADL), Barthel Index for Activities of Daily Living (ADL), SGDS, and Global Deterioration Scale (GDS). Moreover, the amyloid burden was measured at baseline and 3 months after LDRT.
5. Amyloid PET-CT acquisition and PET parameters
18F-florapronol scans were performed to quantitatively assess the amyloid burden before and 3 months after LDRT. For each PET-CT scan, a dose (mean ± standard deviation) of 379.5 ± 12.7 MBq of 18F-florapronol was intravenously injected; furthermore, PET-CT images were acquired from 30 to 60 minutes after injection using the GE Discovery STE 16 (cases 1 and 2) and GE Discovery MI Gen2 (cases 3, 4, and 5) scanners (GE Healthcare, Waukesha, WI, USA).
Four brain regions (frontal, temporal, parietal, and occipital cortices) in each scan were automatically delineated using the MIMneuro application (version 7.16, MIM Software Inc., Beachwood, OH, USA), and the standardized uptake value (SUV) for each region was obtained. Individual SUV ratios (SUVRs) were calculated as the ratio of the mean SUV of each region to the mean SUV of the reference region (cerebellum). The global SUVR was defined as the average SUVR of the four brain regions.
6. Safety evaluation
The safety and tolerability of LDRT were assessed at four time points (weekly during LDRT as well as 1 month, 3 months, and 6 months after completion of LDRT) using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
7. Data analysis
The Wilcoxon signed-rank test was used to analyze the total CDR-SB score as well as the scores for the SNSB domains, K-MMSE-2, CDR-GS, GDS, SGDS, K-IADL, and Barthel-ADL. Additionally, the amyloid burden was measured using 18F-florapronol PET/CT scans before and after LDRT. Statistical analyses were performed using the SPSS software version 24.0 for Windows (IBM Corp., Armonk, NY, USA).
8. Ethical approval and informed consent statements
This study was conducted in accordance with the principles of the Declaration of Helsinki and its amendments or with the laws and regulations of the locality in which the research was conducted (whichever afforded greater protection to the individual). Written informed consent was provided by each patient, the patient’s representative, and by each participating caregiver.
Results
1. Patient population
We enrolled only five patients (all female) with mild-to-moderate AD because patients were no longer enrolled during the recruitment period. All patients completed LDRT and the 6 months of follow-up. Table 1 shows the baseline characteristics of the patients. Patients had a mean age of 71.8 years (range, 60 to 83 years) and mean years of education of 7.8 (range, 3 to 18 years). At baseline, all patients were only treated using acetylcholinesterase (AChE) inhibitors. The mean duration of illness and medication were 30.4 months (range, 8 to 62 months) and 29.2 months (range, 8 to 62 months), respectively. Three of the five patients were apolipoprotein E ε4 carriers.
2. Patient #1
A 75-year-old woman complained of memory disturbance since the age of 73 years. She did not recognize places she had visited several times before. Additionally, she experienced problems with using public transportation by herself, cooking, and shopping. She had graduated from elementary school and was homozygous for ε4 alleles. She had hypertension and a meningioma (0.8 cm) in the left parasagittal dura. She was on several medications, including donepezil (10 mg), choline alfoscerate (400 mg twice a day), amlodipine (5 mg), and alprazolam (0.125 mg). At baseline, the z-scores for SNSB domains were as follows: attention, 1.52; language, -0.71; visuospatial function, -0.25; memory, -2.50; and frontal/executive function, -2.67. The scores for the other neuropsychological measures were as follows: K-MMSE-2, 24/30; SGDS, 8/15; K-IADL, 10/2; CDR, 1 (SB 5); and GDS, 4 (Tables 2, 3).
Just after LDRT, she complained of depressive mood due to impaired cognition; accordingly, vortioxetine 2.5 mg was added to her treatment regimen. At 3 months after LDRT, the donepezil dose was increased to 15 mg due to progressive memory impairment reported by her husband. Despite being more cheerful and participating more in group activities, the neuropsychological tests revealed progressive worsening in all domains except for the frontal and executive function, which showed a z-score improvement from -2.67 to -0.98 at 6 months. No adverse events occurred after the LDRT.
3. Patient #2
A 74-year-old woman complained of progressive cognitive impairment for 2 years. While cooking, she ruined her meals by repeatedly adding salt. Her husband reported episodic memory impairment, including forgetting her daughter’s visit or important appointments. She was apathetic and had no interest in going out or cooking. She had been educated for 6 years and had worked as a farmer. Her mother and older sister also had dementia. She was a carrier of the ε2 and ε4 alleles.
At baseline, the z-scores for each SNSB domain were as follows: attention, -0.31; language, -0.46; visuospatial function, 0.79; memory, -3.31; and frontal/executive function, -0.08. The scores for the other neuropsychological measures were as follows: K-MMSE-2, 20/30; SGDS, 8/15; Barthel-ADL, 20/20; K-IADL, 8/2; CDR 1 (SB 5); and GDS 4 (Tables 2, 3). Her treatment regimen included donepezil (15 mg), choline alfoscerate (400 mg twice a day), and quetiapine (12.5 mg).
The patient completed LDRT without any adverse events. At 3 months after LDRT, she reported improvement in her chronic insomnia. Her husband reported amelioration of her jealousy episodes after LDRT, for example when he was helping neighbors. Further, her daughter reported improvement in recent memory about 6 months after LDRT. There were improvements in all the SNSB domains. Additionally, The K-MMSE-2 and SGDS scores improved from 20 to 23 and from 8 to 2, respectively.
4. Patient #3
An 83-year-old woman with memory impairment was followed up for 6 years in the Department of Neurology. She had been educated for 3 years and had homozygous ε3 alleles. Her medication regimen included donepezil (10 mg) and quetiapine (12.5 mg). Further, she was taking medication for hypertension.
At baseline, she showed impaired language and related functions, memory, and frontal/executive function. The z-scores for each SNSB domain were as follows: attention, 0.32; language and related function, -3.65; visuospatial function, 0; memory, -2.14; and frontal and executive function, -1.71. Moreover, the scores for the K-MMSE-2, SGDS, CDR (SB), and GDS scores were 20/30, 1/15, 1 (4.0), and 4, respectively (Tables 2, 3).
She complained of mild hair loss as an adverse event during LDRT; however, it improved after 6 months. At 3 post-LDRT months, she and her son reported an improvement in mood swings and memory. The CDR (SB) improved from 1 (4.0) to 1 (3.5) at 3 months follow-up. The z-scores for language and related functions, memory, and frontal executive functions improved to -2.56, -1.86, and -1.32, respectively at 6 months follow-up.
5. Patient #4
A 60-year-old woman with a master’s degree who worked as a nurse for 30 years complained of cognitive impairment for 2 years. She made mistakes in calculations and forgot where she placed her belongings. She had recently given up on going to another city by herself since she was worried about losing her way. Her grandmother and father had presented with dementia in their eighties. The patient was heterozygous for ε2/ε4 alleles. She was taking galantamine (8 mg) and fluoxetine (10 mg) due to complaints of depressive mood and loneliness.
At baseline, the z-scores for each SNSB domain were as follows: attention, -0.96; language and related function, -0.26; visuospatial function, -8.9; memory, -4.19; and frontal and executive function, -5.92. Moreover, her K-MMSE-2, SGDS, CDR (SB), and GDS scores were 25/30, 1/15, 1 (4), and 4, respectively (Tables 2, 3).
During LDRT, the patient complained of dizziness, headache, and nausea, which improved after treatment completion. She and her husband reported progressive aggravation of her memory and visuospatial function. She had difficulties solving puzzles, playing with blocks or distinguishing cardinal points. She developed severe depression between 3 and 6 months of follow-up evaluation.
At the 6-month follow-up, the K-MMSE-2, SGDS, CDR (SB), and GDS scores worsened to 19/30, 13/15, 1 (4.5), and 4, respectively. Visuospatial function and frontal executive function showed the most severe worsening, with the z-scores decreasing to -12.97 at 3 months and -24.56 at 6 months, respectively. Her visuospatial function could not be evaluated since she could not complete the Rey Complex Figure Copy task at the 6-month follow-up examination.
6. Patient #5
A 67-year-old woman experienced difficulties finding words and staying on topic for 7 years. She had graduated from elementary school and had heterozygous ε2/ε3 alleles. She was taking donepezil (10 mg) and choline alfoscerate (400 mg) twice daily.
At baseline, the z-scores for each SNSB domain were as follows: attention, -2.65; language and related function, -6.52; visuospatial function, -2.06; memory, -4.10; and frontal and executive function, -4.58. Her K-MMSE-2, SGDS, CDR (SB), and GDS scores were 16/30, 3/15, 1 (5.5), and 4, respectively (Tables 2, 3).
At 6 months after LDRT, her husband reported worsened difficulty in finding words and newly developed fecal incontinence. She did not complete the Rey Complex Figure Copy task; accordingly, the visuospatial function and visual memory could not be evaluated. Her K-MMSE-2, CDR (SB), and GDS scores were aggravated to 11/30, 2 (SB 10), and 5, respectively.
7. Comparison of PET parameters before and after the LDRT
The mean interval between the PET/CT scans before and after LDRT was 101 ± 27.6 days. The mean global SUVRs before and after LDRT were 1.24 ± 0.11 and 1.27 ± 0.14, respectively. Although the global and regional amyloid burden showed an increasing tendency, there were no significant differences between the SUVRs before and after LDRT.
8. Subjective changes
Caregivers of two of five patients reported improved cognitive function and improved memory. For example, patient #3 was confused about going to her house in the apartment after being diagnosed with dementia, but after LDRT, she got better enough to find her house correctly. Then, she was able to take the bus to the grocery store by herself. And, patient #2 came to remember a song she had forgotten in the past, and her short-term memory improved, such as remembering something her husband went to pick up a while ago. Also, the mood of the patient improved to the point where she could joke with the other party.
9. Side effects
One patient complained of nausea (Grade II of CTCAE v5.0) during LDRT, which subsided after treatment. Another patient reported mild hair loss (Grade I of CTCAE v5.0) during LDRT, which also improved after the trial. No other adverse effects were observed.
Discussion and Conclusion
Our findings demonstrated the safety of LDRT in patients with mild-to-moderate AD; however, its efficacy remains unclear.
In a previous case report, an 81-year-old woman with severe AD showed partial restoration of cognitive function and appetite after exposure to several 40-mGy brain CT scans for 2 years [17]. A subsequent pilot study was conducted to confirm the effects of brain CT on patients with severe AD. Four patients with AD underwent three standard brain CT scans, with the first scan emitting a dose of 80 mGy and the subsequent two scans emitting a dose of 40 mGy. Three of the four patients showed qualitative and quantitative improvements in cognition, communication, and behavior [18]. Although this pilot study had several limitations, including a relatively small sample size and lack of placebo groups, it demonstrated the potential for LDRT to treat AD.
Another group recently launched a clinical trial on LDRT for early AD defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria using a LINAC [19]. This study included patients whose florbetapir and FDG PET findings were consistent with AD. Five patients were treated with low-dose whole-brain RT (10 Gy in 5 fractions); among them, four patients showed stability to improvement in the MMSE-2, naming, learning, and memory scores; stability in executive function, processing, mood, and quality of life; and stability to possible improvement in PET imaging. The protocol of 10 Gy in 5 fractions was based on the results of a previous preclinical study [20]. The RT dose was a relatively low total dose of 10 Gy, however, the dose per fraction remained the same as the conventional dose RT (1.8–2 Gy). Notably, several countries such as Germany, extensively apply low total doses with low doses per fraction (total dose 3–6 Gy, single dose <1 Gy) to treat inflammatory-degenerative disorders [9]. There is a mutual relationship between RT and the immune system, which is highly dependent on the dose and quality of radiation. A high total dose of conventional-dose RT (total dose 50–70 Gy, single dose ≥1.8 Gy) generally used to treat cancer exacerbates inflammation [21]; contrastingly, LDRT can be used to control various inflammatory processes and has shown anti-inflammatory properties [22]. Based on the results, the German Society of Radiation Oncology recommends single fraction doses of 0.5–1.0 Gy and total doses of 3.0–6.0 Gy/series for LDRT for painful degenerative skeletal disorders [9]. Accordingly, there has been interest in the efficacy of LDRT for improving inflammation and symptoms in patients with AD. Yang et al. [14] compared the effectiveness of LDRT with 10 Gy and 3 Gy in 5 fractions in an AD mouse model and confirmed that both radiation doses effectively improved cognitive function. The most effective radiation dose for treating AD remains unclear. However, if the treatment effect is the same, it would be advantageous to extensively reduce the radiation dose.
The mechanisms through which LDRT regulates AD remain unclear. In AD, soluble amyloid-β 42, which is abnormally cleaved from an amyloid precursor protein, aggregates into oligomers such as dimers, trimers, pentamers, and fibril to form insoluble amyloid plaques [23]. Soluble amyloid-β 42 oligomers mainly induce neurotoxic effects. In the asymptomatic preclinical stage of AD, microglia clear amyloid-β; however, soluble amyloid-β is overproduced in the more advanced stages, which binds to the surface receptor of microglia and triggers microglial activation, leading to the M1 phenotype [24]. Upon exposure of microglia to stressors such as radiation, the activated M1 microglia release pro-inflammatory cytokines including interleukin-1 (IL-1) beta, IL-6, and tumor necrosis factor-alpha, which contribute to neuronal damage and secondary injury, limiting the utility of the elimination of soluble amyloid-β [25,26]. However, there is increasing evidence that LDRT is neuroprotective rather than an inflammatory stressor. Notably, LDRT has been shown to switch the M1/M2 ratio in the hippocampus of 5xFAD mice. M1-typed and M2-typed microglia produce pro- and anti-inflammatory cytokines, respectively [27]. Specifically, patients with AD present with increased M2-typed microglia in early-stage AD and M1-typed microglia in late-stage AD [28]. Kim et al. [29] reported that lipopolysaccharide- and LDRT-treated BV-2 microglial cells showed a significant increase in the M2 phenotypic marker CD206 compared with LPS- and sham-treated BV-2 cells. Finally, the effect of LDRT on M2 polarization was confirmed by increased TREM2 expression in LPS-induced BV-2 cells.
Although the underlying mechanism remains unclear, numerous cellular and animal studies have reported that low-dose ionizing irradiation (LDIR) promotes longevity, neurogenesis, and cognition while decreasing ROS production [14,20,30,31]. However, the effect of LDRT on microglia in humans remains unclear; moreover, the current understanding of radiation hormesis is poor.
It has been reported that taking donepezil, which is most commonly used for Alzheimer's disease, reduces the MMSE score by an average of 2.5 points per year [32]. Although the follow-up period was as short as 6 months, the present study showed maintained or improved MMSE scores in two out of five patients. In addition, in a recently published study of lecanemab, it was reported that the cognitive decline was delayed by 27% in the experimental group compared to the control group [33]. In our study, cognitive function was improved in 40% of patients, which is quite encouraging, and it is expected that further research will be worthwhile.
This study has several limitations. First, the sample size was too small to determine the efficacy of LDRT. Second, there was no control group. Patients with AD present with cognitive deterioration even with drug treatment. Even if drug treatment slows the rate of cognitive decline, it cannot effectively treat AD. A control group is required to determine whether the addition of LDRT slows down the rate of cognitive decline. Third, the follow-up period (6 months) was too short to evaluate the efficacy of LDRT. Given the long-term progression of AD, follow-up periods of at least 7 months to 4.5 years are required to confirm the reduction of amyloid deposits or symptomatic relief in non-CNS amyloidosis. Contrastingly, our follow-up period of 6 months may have been too short to observe clinical benefits possibly mediated by anti-inflammatory effects. However, since AD is a progressive neurodegenerative disease with rapid progression in old age [34], a practical observation period is required that does not offset the cognitive benefit of intervention.
Currently, six clinical studies into the use of LDIR for the treatment of AD are ongoing according to clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT02769000, NCT05635968, NCT03352258, NCT02359864, NCT03597360, and NCT00599469). Our research team is participating in one of the studies (NCT04203121). In the future, studies on various factors that can affect prognosis, such as stage of AD (mild/moderate/severe), apolipoprotein E genotype, RT energy level (kVp, MeV), RT beam emission type, RT field, total dose, fractional size, intrafractional interval, interfractional interval, dose rate, etc., should be conducted.
In conclusion, at 6 months after LDRT, neurological improvement was seen in 20% of patients. One of our five patients with AD showed an temporary improvement in CDR-SB; moreover, two patients showed stable improvement in the K-MMSE score 2. Since there were no serious side effects above grade 3, LDRT seems to be tolerable in patients with AD. This report is an interim analysis. We are currently under follow-up and will conduct cognitive function tests after 12 months after LDRT (IRB No. 2022-11-022). However, to determine the effect of LDRT in patients with mild-to-moderate AD, a large-scale randomized controlled trial with a long follow-up period of >1 year is required.
Notes
Statement of Ethics
This study was approved by the Institutional Review Board of Chungbuk National University Hospital (IRB No. 2021-04-011-007).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the research grant of the Chungbuk National University Hospital in 2021.
Author Contributions
Conceptualization, Kim A, Seo YS, Yoo MY. Funding acquisition, Kim WD, Seo YS. Investigation and methodology, Kim A. Seo YS, Moon H, Lee JH, Kim C. Project administration, Kim WD, Seo YS. Resources, Seo YS. Supervision, Seo YS, Kim WD. Writing of the original draft, Kim A. Seo YS, Moon H, Lee JH. Writing of the review and editing, Seo YS. Software, Seo YS, Moon H. Validation, Kim A. Seo YS, Moon H, Lee JH. Formal analysis, Kim A. Seo YS, Moon H, Lee JH, Kim C. Data curation, Seo YS, Moon H. Visualization, Seo YS, Kim A.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.