Comparison of sequential versus concurrent chemoradiation regimens in non-metastatic muscle-invasive bladder cancer
Article information
Abstract
Purpose
The treatment approach for non-metastatic bladder cancer is guided by an invasion of the muscular layer of the bladder wall. Radical cystectomy is the recommended treatment for muscle-invasive disease. However, it has considerable morbidity and mortality and is not suited for many patients. Trimodality therapy consisting of chemoradiation after transurethral resection of bladder tumor offers a definitive approach with bladder-sparing potential. However, there is a lack of research defining the optimal combination of chemotherapy and radiation in this setting.
Materials and Methods
We extracted patient data from the National Cancer Database to compare survival outcomes and demographic factors in 2,227 non-metastatic bladder cancer patients who were treated with chemotherapy sequential to or concurrently with radiation. Sequential treatment was defined as chemotherapy beginning >14 days before radiation, and concurrent was defined as beginning within 14 days of the first radiation.
Results
The sequential treatment group patients were younger (mean age, 74 vs. 78 years; p < 0.001) with more advanced disease. We found no difference in overall survival between patients who received chemotherapy sequential to radiation and those who received concurrent chemoradiation only (p = 0.533).
Conclusion
Our data are concordant with a previous prospective study, and support that chemotherapy prior to radiation does not decrease survival outcomes relative to patients receiving only concurrent chemoradiation. Given that the sequential group had an overall higher stage but no difference in survival, downstaging chemotherapy prior to radiation may be helpful in these patients. Further studies including a larger, multi-institutional clinical trial are indicated to support clinical decision-making.
Introduction
Bladder cancer is the sixth most common cancer in the United States, with a median presentation in the eighth decade of life [1,2]. The treatment approach for non-metastatic bladder cancer depends on whether there is an invasion of the muscular layer of the bladder wall [3]. For muscle-invasive, non-metastatic bladder cancer, neoadjuvant chemotherapy followed by radical cystectomy is a standard of care [3]. However, radical cystectomy is a complex procedure with a high risk of potential complications and morbidity. Further, not all patients are appropriate surgical candidates.
An alternative potentially curative modality to radical cystectomy in patients with muscle-invasive, non-metastatic disease is chemoradiation after transurethral resection of bladder tumor [3]. Several studies have supported the efficacy of chemoradiation in muscle-invasive bladder cancer, with cumulative 5-year overall survival data in trials ranging from 49%–73% [4-9]. The BC 2001 trial identified that a 5-fluorouracil/mitomycin C (5FU/MMC) regimen delivered concurrently with radiation significantly improved outcomes relative to radiation alone; 5FU/MMC is now a commonly used regimen for concurrent chemotherapy [10]. The chemoradiation approach has the advantage of being less invasive than surgery and offering the potential to spare the bladder. However, there is a lack of research defining the optimal sequencing of chemotherapy and radiation in this setting.
In past studies, patients frequently received chemotherapy concurrently with radiation [6,8-11] with some studies incorporating a neoadjuvant methotrexate-cisplatin-vinblastine (MCV) chemotherapy regimen prior to radiation [12,13]. Zapatero et al. [14] clinical trial was a small trial of 80 patients with muscle-invasive bladder cancer, in which 41 patients received the MCV regimen followed by 60 Gy radiation to the bladder, and 39 received concurrent cisplatin with 64.8 Gy radiation to the bladder. No significant difference in overall survival was found between the treatment arms; however, patients in the sequential arm did not receive radiation therapy unless they achieved complete response with chemotherapy, therefore the trial did not provide a direct comparison of sequential versus concurrent chemoradiation. Additionally, the trial had several limitations, most prominently the limited number of patients enrolled. There is a need to expand on these data to determine whether the choice to deliver chemotherapy sequential to or concurrent with radiation affects survival. The effect of patient demographic and disease-specific factors on outcomes in these two treatment arms also has not been widely examined.
To address these gaps in knowledge, we extracted patient data from the National Cancer Database (NCDB) to compare patients with non-metastatic bladder cancer who were not surgical candidates and treated with chemoradiation sequentially or concurrently. Our primary objective was to compare patient demographic factors and tumor stage between patients who received chemotherapy prior to or concurrent with radiation, to determine whether these factors were associated with the treatment regimen. Our secondary objective was to compare survival outcomes in patients treated with upfront chemotherapy prior to radiation treatment, or concurrent radiation with chemotherapy to help outline best practices for non-surgical candidates in this setting.
Materials and Methods
1. Data source and population
Data for this study were extracted from the 2018 NCDB, a clinical oncology database sourced from hospital registry data that are collected at more than 1,500 Commission on Cancer accredited programs. Overall survival is defined as the number of months from the date of initial diagnosis to the date of last contact or death due to any cause. Two treatment strategies are defined based on the sequence of chemotherapy and radiation. Treatment 1 is defined as a sequential treatment in which chemotherapy start date is more than 14 days prior to radiation start date (180 days prior to radiation as maximum). Treatment 2 is defined as a concurrent treatment in which chemo start date is started within 14 days of radiation start date (before or after). A total of 671,462 patients were enrolled for bladder cancer between 2004 and 2008. After the inclusion/exclusion criteria (Fig. 1), 3,064 patients remained. After definition of the treatments, another 837 patients did not fit into either treatment group, leaving 2,227 patients included in this analysis.
2. Demographic and clinical staging information
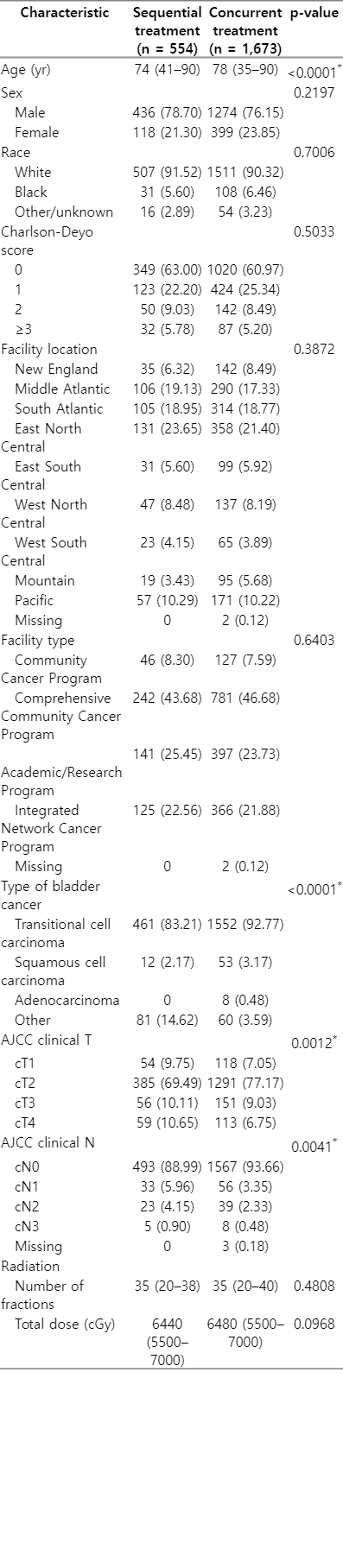
Age at diagnosis, sex, race, Charlson-Deyo score, facility location, and facility type were considered as patient demographic information and facility information. Race was categorized as White, Black, and other/unknown. The Charlson-Deyo score is a weighted score derived from the sum of the scores for each of the comorbid conditions listed in the Charlson Comorbidity Score mapping table [15]. The American Joint Committee on Cancer (AJCC) clinical staging information from the AJCC 7th edition is compared between treatments and survival time. Staging information was divided into clinical T staging based on histology and clinical N staging based on lymph node involvement.
3. Statistical analysis
Patient demographic and facility information were summarized by treatment strategies using medians and ranges for continuous variables and frequencies and percentages for categorical variables. Based on the Shapiro-Wilks test, continuous variables did not fit a normal distribution, so the Wilcoxon two-sample test was applied to compare the difference between the two treatment strategies. The chi-square test was used to compare categorical variables by treatment strategy. The Kaplan-Meier method and log-rank tests were used to estimate overall survival distributions among groups defined by: two treatments (sequential, concurrent); age (>77, ≤77 years); clinical T stage (T1-2, T3-4); clinical N stage (N0, N1-3); clinical N stage (N0-1, N2-3); and Charlson-Deyo score (0, 1, 2, ≥3). Cox proportional hazard model was conducted to explain the relationship between the two treatments and overall survival, while adjusting for age, sex, race, clinical T stage, clinical N stage, and Charlson-Deyo score. The proportional hazard assumption was tested to show the hazard function was proportional over time using a supremum test. If a p-value was larger than 0.05 for each variable, the model satisfied the proportional hazard assumption. If the proportional assumption was violated, stratification analysis would be conducted. Interactions between treatment strategy and covariates were considered in the Cox regression model. If the interaction was significant (p < 0.05), then it was kept in the model; and a backward model selection method will be used to select the best model.
Results are presented as estimates, hazard ratio (HR), 95% confidence interval, and p-values. p-values <0.05 are considered statistically significant. All statistical analyses were undertaken using SAS Software version 9.4 (SAS Institute Inc., Cary, NC, USA).
For matching analysis, pairs between sequential and concurrent treatment patients were constructed using greedy nearest neighbor matching on the logit of the propensity score 1:1 without replacement. Covariates in Table 1 such as age, sex, race, Charlson-Deyo score, facility location, facility type, type of bladder cancer, and AJCC clinical T, N stage were included during the matching. After matching, a dataset with a sample size of 1,108 was created, with 554 patients in the sequential group and 554 patients in the concurrent group. Then, Kaplan-Meier estimate and a coxed model, including treatment group, age, sex, race, clinical T/N stage, Charlson-Deyo score, type of cell, and interaction term between type of cell and treatment group was constructed based on the matched data.
Results
Table 1 shows the breakdown of demographic and staging characteristics between patients receiving concurrent chemoradiation or sequential chemotherapy followed by radiation. Patients in the sequential treatment group were found to be significantly younger than those in the concurrent group (Median age 74 years vs 78 years, p<0.001). Most patients in both groups had transitional cell carcinoma, however, the differences in the breakdown of distribution of cancer type between the two groups were significant. All the patients with adenocarcinoma (8/8) were in the concurrently treated group, and 81% (53/65) of the patients with squamous cell carcinoma were in the concurrently treated group. No significant differences were noted in sex, race, Charlson-Deyo score, treatment facility location, or facility type between the two groups. There was also no significant difference in the total dose of radiation or number of fractions received between the two groups. Mean and median times from the start of chemotherapy to the start of radiation therapy in the sequential group were 85.4 days and 84 days, respectively, with a range from 15–180 days.
Patients in the sequential group were significantly more likely to have greater clinical T stage than patients in the concurrent group (clinical stage T4: 10.65% of patients treated sequentially vs. 6.75% of patients treated concurrently, p = 0.001) (Table 1). Sequentially treated patients were also significantly more likely to have nodal metastases than patients treated concurrently (p = 0.004).
There was no significant difference in overall survival between the two treatment groups (p = 0.533) (Fig. 2A); median time to death or last contact was 27.9 months in the sequential group and 25.2 months in the concurrent group. In an adjusted Cox proportional hazard regression model, the risk of death in the sequential treatment group was not significantly different than that in the concurrent group (HR = 1.336, p = 0.297) (Table 2). Age, sex, race, or histology were not significantly associated with overall survival (Table 2). The only factor significantly associated with overall survival was the Charlson-Deyo score; scores of 0-2 were associated with a significantly decreased risk of death relative to scores ≥3 (Table 2, Kaplan-Meier estimate for Charlson-Deyo score shown in Supplementary Fig. S1). To determine whether survival was equivalent when adjusting for differences in demographic and disease stage variables between groups, a matching analysis was performed to generate paired patients between groups matched by age, sex, race, Charson-Deyo score, facility location, facility type, type of bladder cancer, and AJCC clinical T and N stage. A Kaplan-Meier estimate (Fig. 2B) and Cox proportional hazard regression model (Table 3) were performed on the matched groups (Fig. 2B and Table 3, respectively). The results were consistent with the unmatched Kaplan-Meier and Cox model results; there was no significant difference in overall survival between the two treatment groups after matching analysis (p = 0.539).
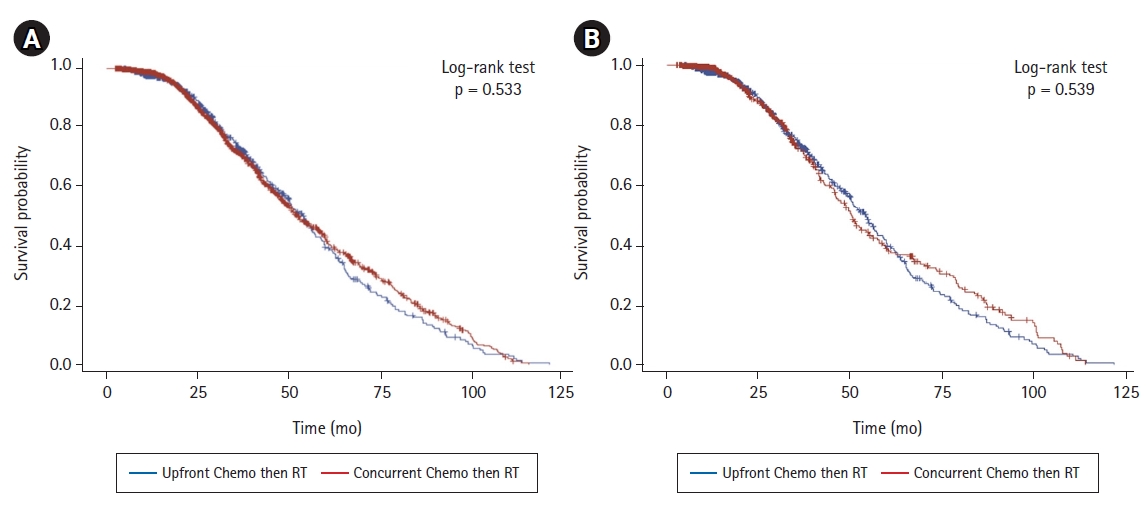
(A) Kaplan-Meier curve of overall survival time in patients with non-metastatic bladder cancer treated with either sequential chemotherapy followed by radiation therapy (Upfront Chemo then RT, blue line, n = 554) or concurrent chemotherapy and radiation therapy (Concurrent Chemo then RT, red line, n = 1,673). (B) Kaplan-Meier curve of overall survival time after matching (Upfront Chemo then RT, blue line, n=554; Concurrent Chemo then RT, red line, n = 554).

Cox model of overall survival and treatment, adjusted for age, sex, race, clinical stage and Charlson-Deyo, histology and the interaction between treatment and histology
Discussion and Conclusion
Our analysis found no difference in overall survival between patients who received chemotherapy prior to radiation and those who received concurrent chemoradiation only. While we were unable to exclude patients in the sequential group who continued to receive chemotherapy during radiation, our data still allow for a comparison of the effect of neoadjuvant chemotherapy on survival in this setting. Our data demonstrate that chemotherapy administered prior to radiation does not decrease survival outcomes relative to patients receiving only concurrent chemoradiation.
Our study represents the first retrospective review of patient data comparing concurrent versus sequential chemoradiation in bladder cancer and involved an analysis of a database containing over 670,000 bladder cancer patients. Findings from our study are concordant with the earlier clinical trial [14]; both studies showed no difference in overall survival outcome. The Zapatero study [14] was limited by small patient numbers and by unequal treatments between groups but was prospective in nature. While our study was limited by its retrospective nature, it involved large numbers of patients and therefore provides a comprehensive analysis.
The sequential group was more likely to have positive lymph nodes and had a more advanced disease stage. Since systemic therapies administered neoadjuvant to local ablative therapy are used in patients with a greater likelihood of distant metastases, upfront chemotherapy prior to radiation could have been given in this group with the intent of ablating possible micrometastatic disease. Our data demonstrate equivalent survival in these patients despite more advanced disease relative to the concurrent chemoradiation-only group. This finding supports the efficacy of neoadjuvant chemotherapy prior to radiation in this patient cohort. However, even when matching patients between groups to adjust for differences in demographic and disease stage variables, there remained no difference in overall survival between the sequential and concurrent groups. To better determine whether more advanced tumor and lymph node stages preferentially benefit from sequential chemoradiation, further study is needed, including a multi-institutional, prospective trial in which patients are stratified by tumor and lymph node stage.
Notably, a proportion of the advanced disease patients in the sequential group may have received both neoadjuvant downstaging chemotherapy and concurrent chemoradiation as per National Comprehensive Cancer Network guidelines, which was not assessable through the NCDB database. There remains a need for further study to determine the efficacy of concurrent chemoradiation in the setting of patients having received neoadjuvant chemotherapy. The previous BC 2001 trial demonstrated a clear advantage of concurrent chemoradiation vs radiation alone, with or without neoadjuvant chemotherapy [10]. This trial demonstrated a survival advantage of chemotherapy concurrent with radiation, with a HR of 0.62 with both neoadjuvant and concurrent chemoradiation relative to radiation alone, and a HR of 0.71 with concurrent chemoradiation relative to radiation alone [10]. Importantly, the HR was decreased regardless of whether neoadjuvant chemotherapy was given, suggesting concurrent chemotherapy to be efficacious regardless of whether the patient received neoadjuvant chemotherapy. The lower HR with neoadjuvant suggests there could be a greater benefit to giving both neoadjuvant and concurrent chemotherapy with radiation.
In addition to the difference in disease stage between groups in our analysis, the sequential group was also significantly younger. Neoadjuvant platinum-based chemotherapy is standard-of-care for the surgical approach [16], and younger patients are more likely to be considered surgical candidates. Therefore, it could be that a proportion of sequentially treated patients were initially intended as surgical candidates, and received neoadjuvant chemotherapy in preparation for surgery, but later opted for radiation treatment as a bladder-sparing approach. A future study using a database with information on the intent of treatment could provide more information on clinical decision-making related to patient demographic and disease factors, and their effect on patient outcomes.
The only factor in our analysis found to significantly affect survival was Charlson-Deyo comorbidity index. A Charlson-Deyo score of 3 or greater was associated with decreased survival, relative to a score of 2 or less. This is consistent with literature reports; comorbidities, as determined by the Charlson index or by other measures of comorbidity level, have been found to independently predict survival in bladder cancer patients with the non-invasive or invasive disease [17,18]. While the Charlson-Deyo score is adjusted for age, age itself was not found to affect overall survival in either treatment group in our analysis. This highlights the morbidity and mortality of bladder cancer. With cancers in which patients are likely to die of their cancer rather than with their cancer, there is less skewing of overall survival data by patients who die of other age-related causes. Additionally, bladder cancer is known to be associated with patients with poor general health and significant smoking history which directly affect patient survival regardless of their bladder cancer diagnosis. Measures of cancer-specific survival, while not possible in our analysis should be performed in future studies to exclude non-cancer causes of mortality from survival calculations.
A further limitation of our study is the inability to evaluate disease-free survival or response rate since these data were not available in the database. We recommend a future multi-institutional clinical trial enrolling a larger patient number, designed as a non-inferiority study, and using a chemotherapy regimen that is standardized between treatment arms to guide clinical decision-making. Future clinical trials should include an evaluation of overall survival and various parameters of disease-free survival and disease progression.
In conclusion, evidence from our study and previous studies supports no difference in overall survival between patients with non-metastatic bladder cancer who received chemotherapy prior to radiation and those who received concurrent chemoradiation only. That no survival difference was found despite overall poorer prognostic features in the sequential patient group suggests downstaging chemotherapy prior to radiation may be advantageous for higher stage patients. However, further studies using more detailed database analysis and large clinical trials are needed to outline best practices for these patients.
Notes
Statement of Ethics
This study was conducted using national registry data, therefore written informed consent was not required.
Conflict of Interest
Benjamin A. Teply reports funding for clinical research from Bristol-Myers-Squibb, and Advisory Board Consultancy for Seagen and Astra Zeneca. Raymond C. Bergan is on the Scientific advisory Committee for the National Cancer Institute, Northwestern University, and University of Arizona. All other authors have no conflicts of interest to declare.
Funding
None.
Author Contributions
Conceptualization, Enke CA, Bergan RC, Teply BA, Baine MJ. Investigation and methodology, Vieira HM, Kasper DP, Wang R. Supervision, Baine MJ. Writing of the original draft, Vieira HM, Kasper DP. Writing of the review and editing, Enke CA, Bergan RC, Teply BA, Baine MJ. Statistical analysis, Wang R, Smith LM.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2023.00262.
Kaplan-Meier curve of overall survival time in patients with non-metastatic bladder cancer based on Charleson-Deyo score (n = 2,227).