Introduction
Ependymoma is a relatively rare brain tumor which occurs about 9% in children and 3% in adults out of all primary brain tumors [1]. In adults 75% of ependymomas develop in the spinal canal whereas 90% of ependymomas are intracranial tumors in children. Brain ependymal tumors are categorized into subependymoma (World Health Organization [WHO] grade I), mixopapillary ependymoma (WHO grade I), ependymoma (WHO grade II), and anaplastic ependymoma (WHO grade III) according to the WHO classification (2007) [2]. Marseille grading system which classifies tumors as low grade (0-1 factor) and high grade (2-3 factors) according to tumor necrosis, microvascular proliferation, and mitosis (<5 vs. Ōēź5 in 10 consecutive high-power fields) is also used.
The treatment option for ependymoma is surgical resection followed by radiotherapy, although there are no randomized prospective studies available yet [3-6]. Up to this date, various prognostic factors for determining the extent, dose and risk of recurrence factors of radiation therapy has gone under analysis, but nothing has been clearly defined.
This study has been performed in order to evaluate the patterns of failure, survival rate, treatment-related toxicity and prognostic factors for patients with ependymoma receiving postoperative radiotherapy.
Materials and Methods
1. Patient population
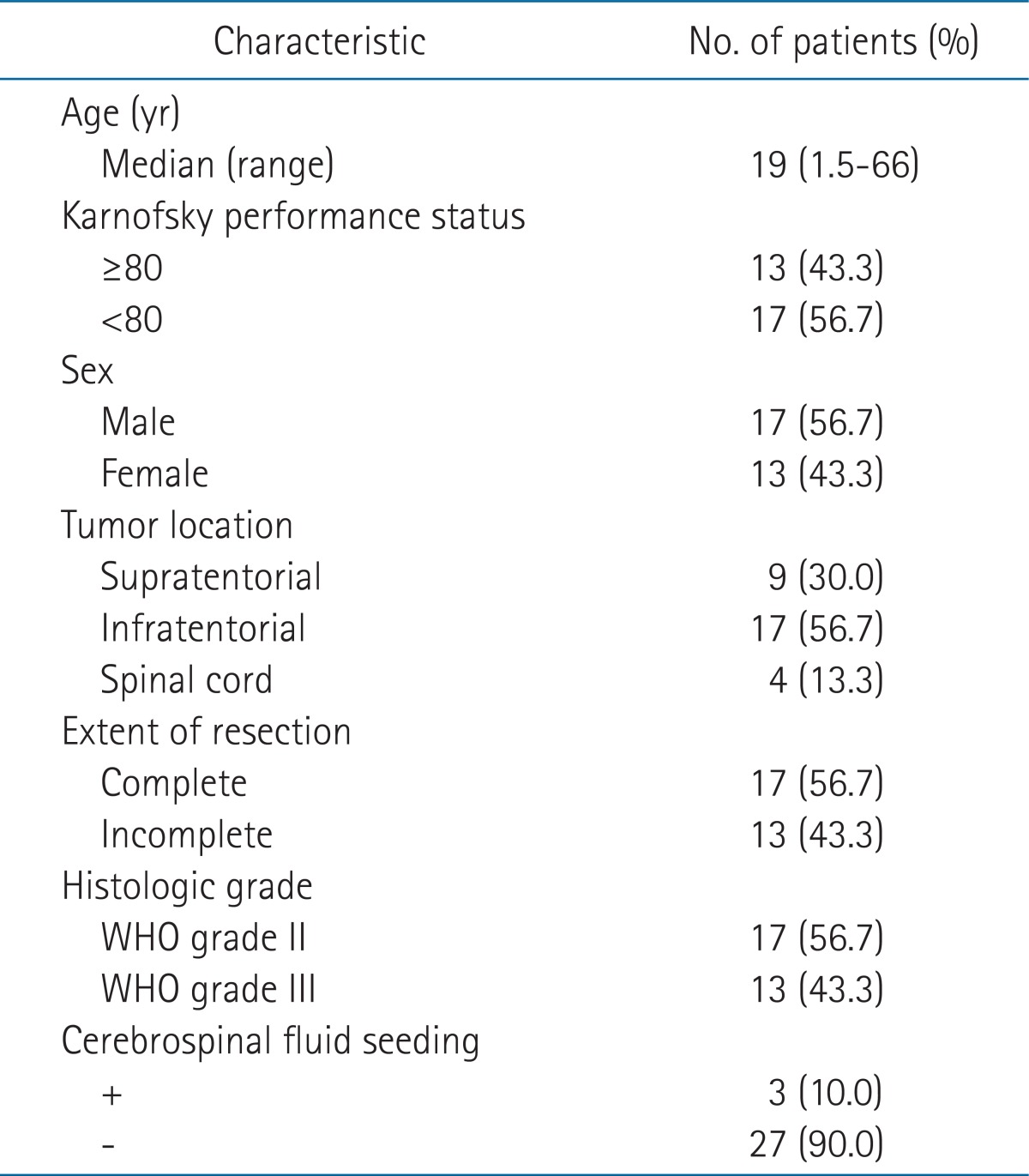
Thirty patients who have undergone surgery and postoperative radiotherapy for ependymoma between the period of June 1994 and June 2008 were reviewed retrospectively. We excluded the following patients; WHO grade I ependymoma, double primary cancer, recurrent ependymoma, or previous history of radiation to brain or spinal region. The age of patients ranged from 21 months to 66 years (median, 19 years) where 15 patients were under the age of 18 and among them 5 were younger than three years old (Table 1). The number of male and female patients was 17 and 13, respectively. Thirteen patients had Karnofsky performance status (KPS) higher than 80, which was less than majority. All patients underwent brain computed tomography (CT), magnetic resonance imaging (MRI), and cerebrospinal fluid (CSF) analysis. Three (10%) patients showed CSF seeding with positive on CSF cytology and among these three patients, 2 had WHO grade II ependymoma while the remaining patient had WHO grade III anaplastic ependymoma.
2. Tumor location, extent of surgery, and tumor grade
Twenty-six (86.7%) patients had intracranial tumor, and the rest four (13.3%) patients had tumors in the spinal canal. Nine (30%) patients had tumor involving the supratentorial region and 17 (56.7%) in the infratentorial region. Surgery was performed with the aim of gross total resection. The extent of resection was categorized as complete or incomplete resection and included 17 (56.7%) and 13 (43.3%) patients, respectively. Seventeen patients had grade II ependymoma while 13 had grade III anaplastic ependymoma according to the WHO grading system.
3. Radiotherapy and chemotherapy
All patients received three dimensional conformal radiotherapy except one, and the postoperative irradiation was performed with 4 or 6 MV photon beam. Clinical target volume (CTV) was defined as 1.5 cm extension from the tumor bed and planning target volume (PTV) margin was added 5 mm from the CTV. Total dose of 54 Gy in 1.8 Gy-fractions was delivered for patients who underwent complete resection and 59.4 Gy for those with incomplete resection, but there was no difference in the total dose delivered for tumors of different WHO grading system. The median for actual irradiated dose was 52.8 Gy (range, 45 to 63 Gy). The extent of radiation therapy was limited to the primary tumor bed for 26 patients with primary tumor only while craniospinal irradiation (CSI) was performed for 3 patients with CSF seeding and for 1 patient with anaplastic ependymoma in spinal canal received whole spine irradiation. Five patients received postoperative chemotherapy, among those, 3 patients were under the age of 3 who received chemotherapy with cyclophosphamide, vincristine/cisplatin, etoposide before radiotherapy, while the rest 2 patients above 18 years of age received chemotherapy after radiotherapy. The median interval period between surgery and radiotherapy was 33 days (range, 21 to 71 days) except for 3 patients who received postoperative chemotherapy before radiotherapy.
4. Follow-up and statistical analysis
All patients were examined at least once a week during the course of radiotherapy for assessment of acute toxicities. After the completion of treatment, patients were followed up every 1-3 months for the first year, 3-6 months for the following 2 years, and every 6-12 months after then. Progression was evaluated with CT, MRI, and tissue confirmation. Median follow-up period for all patients was 51 months (range, 12 to 172 months) and for survivals was 58 months (range, 25 to 172 months). Progression-free survival (PFS) and overall survival (OS) were estimated from the date of surgery with the Kaplan-Meier method. The impact of clinical and therapeutic variables on survival was evaluated by comparing entire OS curves using the log-rank test. Probability (p) values < 0.05 were considered significant.
Results
Fourteen (46.7%) out of 30 patients experienced recurrence and median progression free duration was 35.5 months (range, 4 to 167 months). Twelve of 14 patients who experienced recurrence died. Ten out of these 12 patients were due to disease progression while other 2 patients were from respiratory failure and aspiration pneumonia and they each experienced re-surgery or Gamma-knife radiosurgery for recurrent disease. One of 2 patients who remained alive after recurrence underwent re-surgery for recurrent lesion at the primary infratentorial site, and is still alive without disease. Another patient received additional complete resection and postoperative radiotherapy for recurrent spinal lesion outside the primary site. However, at 22nd month of disease-free period, this patient developed a new recurrent spinal seeding mass. Chemotherapy with cyclophosphamide, vincristine/cisplatin, etoposide was delivered and this patient is currently alive with stable disease.
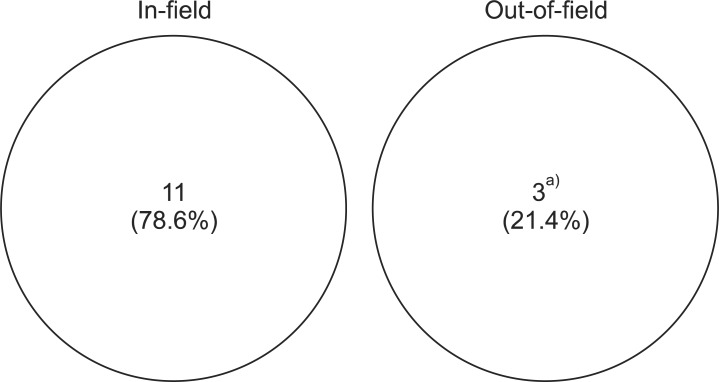
By classification of 14 recurrent cases based on the treatment field, 11 developed within the treatment field while 3 were out-of-field recurrence (Fig. 1). All three out-of-field recurrent cases were due to CSF seeding, where was originated from infratentorial ependymoma, infratentorial anaplastic ependymoma, and spinal anaplastic ependymoma, respectively. These patients were absent from CSF seeding at the period of initial diagnosis. All three patients who had disease with CSF seeding at the initial diagnosis received craniospinal irradiation. Among them, 2 patients experienced recurrence within the treatment field, one at the primary site while other in the spinal canal. Nine out of 14 recurred cases were anaplastic ependymoma, and other 5 were ependymoma. In tumor histological point of view, the progression rate was 69.2% for 13 cases of anaplastic ependymoma and 29.4% in 17 cases of ependymoma. Seven (41.2%) patients among 17 who underwent complete resection and 7 (53.8%) among 13 who underwent incomplete resection experienced recurrence.
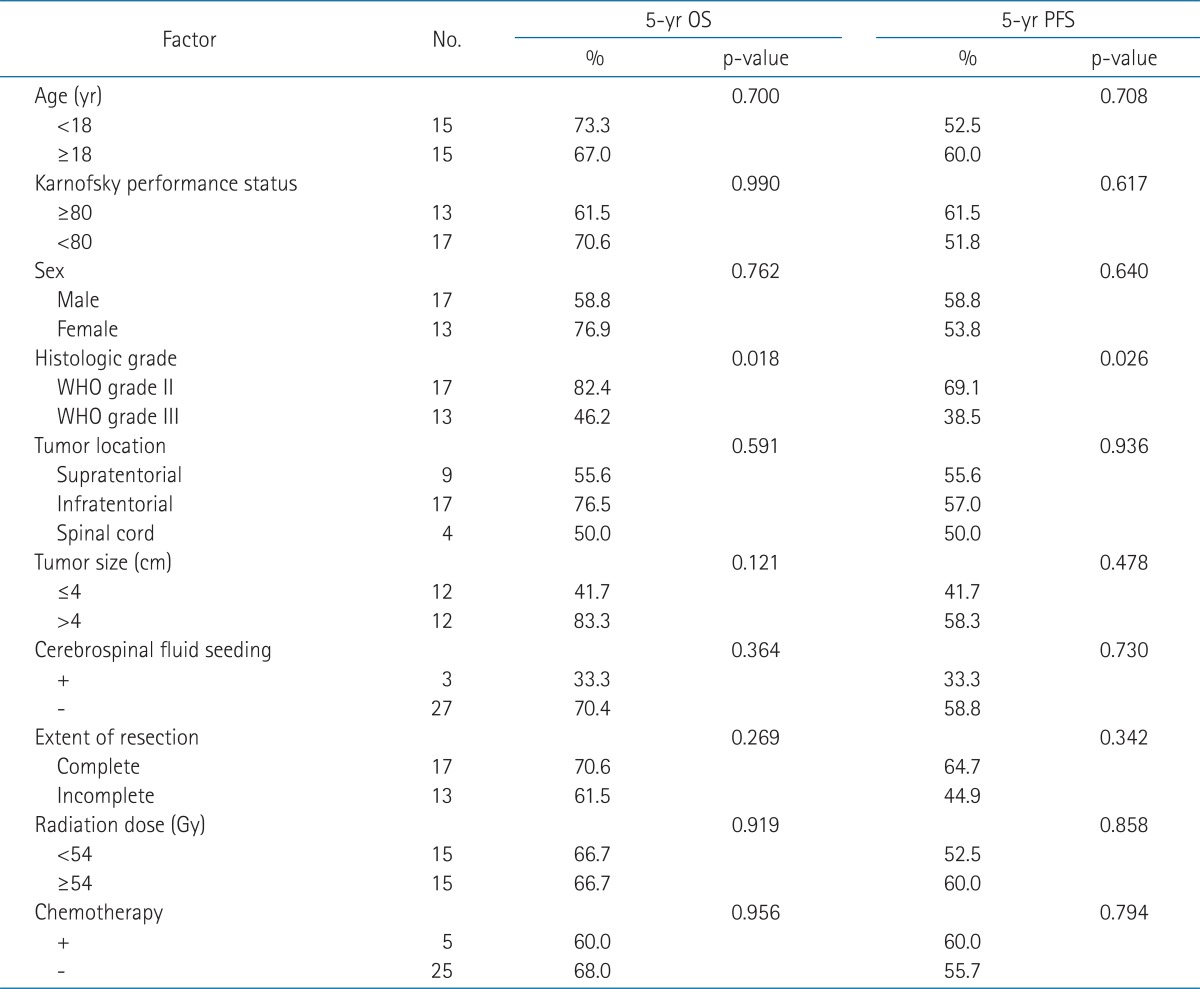
The 5-year OS and PFS rates were 66.7% and 56.1%, respectively, and median OS and PFS were 74.5 and 35.5 months, respectively (Fig. 2). On univariate analysis, histological grade was a statistically significant prognostic factor for OS and PFS (Table 2). Five-year OS of 82.4% for the patients who had ependymoma was superior to that of 46.2% for those who had anaplastic ependymoma. Five-year PFS was also superior in patients who had ependymoma (69.1% vs. 38.5%) (Fig. 3). However, age, KPS score, sex, tumor location, tumor size, presence of CSF seeding, extent of resection, radiation dose, or chemotherapy were not statistically significant factors affecting the survival.
Complications observed after the surgery and postoperative radiotherapy included short stature, facial palsy on the left side, and paraplegia. The cause of facial palsy was not found but it improved spontaneously even without treatment. Possible cause of paraplegia is due to disease progression. There was no other severe complication such as brain necrosis.
Discussion and Conclusion
This study evaluated selective patients who underwent surgery and postoperative radiotherapy, and whose 5-year PFS and OS rates were 66.7% and 56.1%, respectively. The results of present study were similar to those of previous studies that 5-year PFS and OS rates were 62-84% and 40-63%, respectively [7-10]. The OS and PFS rates increased slightly to 70.4% and 58.8%, respectively, if three patients with CSF seeding at the time of initial diagnosis were excluded. However, even in case where significant portion of anaplastic ependymomas were included, the finding showed comparable result to previous ones. By and large, 11 out of 14 recurrent cases showed local recurrence within the treatment field. There are many prognostic factors affecting progression, including local recurrence and survival, i.e., histologic grade, extent of surgery, tumor location and presence of postoperative radiotherapy. However, in this study histologic grade was the only significant factor.
For ependymoma and anaplstic ependymoma 5-year OS were 82.4% and 46.2%, and 5-year PFS were 69.1% and 38.5%, respectively. This indicates that the histologic grade of tumors has a significant effect on the survival rate. Similarly other studies including a multi-institutional retrospective study of intracranial ependymoma in children and St. Jude Children's Research Hospital showed that the prognosis of anaplastic ependymoma was worse than that of other histologic grade [11,12]. Metellus et al. [8] also reported that the 5-year OS of ependymoma (93.8%) was superior to that of anaplastic ependymoma (62.1%). Furthermore, several other studies have shown that the histologic grade of tumor is an important factor affecting OS or the PFS [7,10,13-16].
The extent of resection was not a statistically significant prognostic factor, in our study but it might be due to small sample size. On the contrary to present result, the extent of resection achieves recognition as a significant prognostic factor in many studies [8,9,13,14,17]. First of all, Timmermann et al. [17] analyzed several prognostic factors that influence survival, where 3-year PFS rate was 83.3% in complete resection group and 38.5% in incomplete resection group with statistical significance. Additionally, Metellus et al. [8] showed the importance of the extent of resection as a prognostic factor and recommended addition surgery to residual lesion for complete resection, if possible. Although present study did not show statistical significance in relation to the extent of surgery, four patients with anaplastic ependymoma, who had undergone incomplete resection developed recurrence and all of them died within 2 years. This suggests the importance of the extent of resection.
There have been numerous studies on examination of association between tumor location and prognosis. Metellus et al. [8] reported that the survival rate of infratentorial located tumors is superior to that of supratentorial tumors. Although the results of the study by Korshunov et al. [13] of 258 patients with ependymoma and anaplastic ependymoma did not demonstrate the significance of tumor location as prognostic factor, subgroup analysis with 127 patients who had anaplastic ependymoma showed better 5-year PFS and OS rates, 43% and 75%, respectively for infratentorial tumor than 8% and 51%, respectively for supratentorial tumor. On the other hand, Needle et al. [18] insisted on the superior survival of supratentorial tumor due to possibility of easier resection, and Timmermann et al. [17] also demonstrated no significant difference in prognosis in relation to tumor location. Hence, the significance of tumor location in prognosis is still a controversial issue.
Subgroup analysis based on age groups (<18 or Ōēź18) showed no relevant relationship between age and survival in our study. Moreover there are limited studies attempting to compare the prognosis of children and adult groups, therefore, it is not possible to draw a conclusion as to which subgroups have better survival. However, on the contrary to our study, Korshunov et al. [13] demonstrated difference in the survival rate between age groups. Five-year PFS and OS rate of 18% and 52%, respectively was observed in patients under 16 years of age and this was worse compared to the group of patients of age 16 and above (5-year PFS and OS, 34% and 74%, respectively) [13].
Initial studies on ependymoma pointed out the risk of CSF seeding, but with the development of imaging technologies, recent studies showed that isolated spinal canal recurrence was uncommon even with the omission of prophylactic CSI [19]. Hence involved field irradiation has become the standard procedure for postoperative radiotherapy for cases where there is no evidence of CSF seeding [17,20-22]. This present study agrees with the above mentioned results since it was found that most recurrences occurred within the treatment field and CSF failure was rarely observed. Thus, boost with stereotactic radiosurgery (SRS) after conventional postoperative radiotherapy should be consider for patient at high risk for recurrence, i.e., anaplastic ependymoma and incomplete resection. Five children diagnosed with either ependymoma or anaplastic ependymoma at St. Jude Children's Hospital who received postoperative radiotherapy with total dose of 50.4-55.8 Gy, received SRS with dose of 9-15 Gy since residual disease of less than 3 cm was observed. Of these children, 4 patients showed 15-40 months of PFS rate while three showed complete remission [23]. Four children that showed PFS had received SRS within 30 days of conventional radiation treatment, and one child who died due to progression of disease had received SRS 6 months after the radiation therapy. The Korean Society for Pediatric Neuro-Oncology (KSPNO) protocol for ependymoma recommends additional SRS with dose of 6 Gy to be delivered to those tumors with postoperative residual disease of less than 3 cm or those with good response to chemotherapy even with residual disease of greater than 3 cm.
Ependymomas showed relatively good survival rate with surgery and radiation therapy. Additionally depending on the histologic grade, difference in the OS and PFS showed meaningful result. Almost all recurrences occurred within the primary tumor site, thus we suggest further evaluation on the intensity-modulated radiotherapy or stereotatic radiosurgery for high-risk patients such as those with anaplastic ependymoma.