Total lymphoid irradiation based conditioning for hematopoietic stem cell transplantation in severe aplastic anemia
Article information
Abstract
Purpose
To retrospectively evaluate the outcome and toxicity of total lymphoid irradiation (TLI) based conditioning regimen for allogeneic hematopoietic stem cell transplantation (HSCT) in severe aplastic anemia (SAA) patients who experienced an engraftment failure from prior HSCT or were heavily transfused.
Materials and Methods
Between 1995 and 2006, 20 SAA patients received TLI for conditioning of HSCT. All patients were multi-transfused or had long duration of disease. Fifteen (75%) patients had graft failure from prior HSCT. In 18 (90%) patients, the donors were human leukocyte antigen identical siblings. The stem cell source was the peripheral blood stem cell in 15 (75%) patients. The conditioning regimen was composed of antithymocyte globulin plus TLI with a median dose of 750 cGy in 1 fraction. The graft-versus-host disease (GVHD) prophylaxis used cyclosporine with methotrexate.
Results
With a median follow-up of 10.8 years, graft failures developed in 6 patients. Among them, 3 patients received their third HSCT to be engrafted finally. The Kaplan-Meier overall survival rate was 85.0% and 83.1% at 5 and 10 years, respectively. The incidence of acute and chronic GVHD was 20% and 20%, respectively. None of the patients have developed a malignancy after HSCT.
Conclusion
In our study, TLI based conditioning in allogeneic HSCT was feasible with acceptable rates of GVHD in SAA patients who experienced graft failure from prior HSCT or was at a high risk of graft rejection. We achieved relatively better results of engraftment and survival with a long term follow-up.
Introduction
Aplastic anemia is an unusual hematologic disorder characterized by bone marrow hypocellularity and peripheral blood pancytopenia. Immunosuppressive therapy or hematopoietic stem cell transplantation (HSCT) is the main treatment modality for severe aplastic anemia (SAA), based on patient's age, availability of histocompatible siblings, and disease severity. The HSCT restores normal hematopoiesis and is accepted as the therapy of choice for SAA patients who have human leukocyte antigen (HLA) matched donors. Although the survival rate has been improved, the major causes of failure after HSCT in SAA are graft-versus-host disease (GVHD), infection, and graft rejection. Recently, mortality dropped with the decreased rate of GVHD related deaths, which coincided with the introduction of cyclosporine, however, less so in infection and graft failure. In particular, the risk of graft failure is associated with older patients with exposures of large numbers of transfusions and long duration of the disease [1-3]. Several preparative regimens have been utilized in an attempt to decrease graft rejection in HSCT. These regimens include increased cell administration or increased immunosuppressive potential of the regimen, such as adding radiotherapy to cyclophosphamide or using multi-agent chemotherapeutic regimens [4-7].
Radiotherapy-based conditioning in HSCT is usually a total body irradiation (TBI) [8]; yet, in order to reduce the significant morbidity, total lymphoid irradiation (TLI) was introduced on the basis of studies in animals that suggested that TLI could be used successfully in order to condition the HSCT recipients [9,10]. After Ramsay et al. [4] first reported in 1980 that a single dose TLI plus cyclophosphamide prior to HSCT resulted in a low rate of graft rejection and GVHD in 9 SAA patients, several studies have showed favorable results using TLI or thoraco-abdominal irradiation (TAI) conditioning for HSCT in SAA patients in the early 1990s [5-7]. Although radiotherapy based conditioning in HSCT has been reported to be associated with a higher risk of late complication, in particular, secondary cancers, GVHD, infertility, and pulmonary toxicity [11-13], adding radiation to the conditioning regimen can effectively enhance the immunosuppressive potential of HSCT and reduce the graft failure rate in SAA patients with a high risk for graft rejection, who were heavily alloimmunized from multiple transfusion or experienced graft failure on prior allogeneic HSCT.
This study evaluated the outcome and toxicity of TLI-based conditioning regimen for HSCT in SAA patients of high risk for graft rejection in our institution.
Materials and Methods
1. Patients
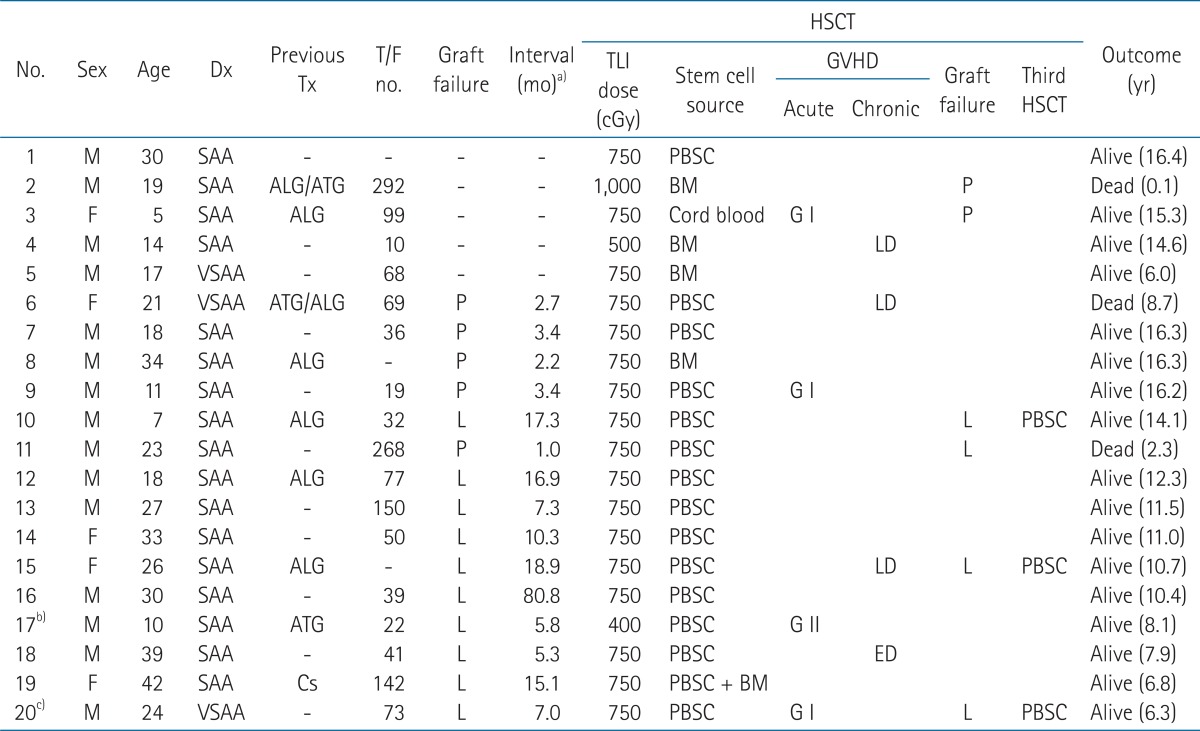
A total of 20 patients with SAA received TLI for conditioning of HSCT in our institution between June 1995 and April 2006. The patient's characteristics are listed in Table 1. The patients' age ranged between 5 and 42 years (median, 22 years). There were 15 males and 5 females. The median follow-up was 10.8 years (range, 0.1 to 16.4 years). All patients had history of multiple transfusions, and/or had a period of disease prior to HSCT in our institution for 3 years or longer. Fifteen (75%) patients received more than 20 units of transfusions (red blood cells and/or platelets), and 4 (20%) patients received more than 100 units of transfusions. Nine (45%) patients failed to respond to the previous immunosuppressive therapy. Fifteen (75%) patients experienced engraftment failure from prior allogeneic HSCT, and the interval between the first and second HSCT was a median of 7 months (range, 1 to 81 months).
Prior to therapy, diagnoses were confirmed by aspirates and biopsies of bone marrow. SAA was defined according to the criteria of the International Aplastic Anemia Study Group [14]. These criteria require the presence of at least two of the following three peripheral blood findings: 1) an absolute neutrophil count (ANC) of less than 0.5 × 109/L; 2) a corrected reticulocyte of less than 1%; and 3) a platelet count less than 20 × 109/L. The bone marrow (BM) must be hypocellular with lymphoid cells usually predominated. Aplastic anemia was considered very severe if it met the criteria for severe disease, and the ANC was less than 0.2 × 109/L.
Patients or their legal guardians signed the informed consent for HSCT. Data collection, storage, and analysis were all conducted in compliance with the hospital's Institutional Review Board.
2. Donors
All donors were matched for HLA-A, -B, and -DR with the recipient patients: HLA identical siblings in 18 cases, matched related donor (patient's father) in 1 case and matched unrelated donor in 1 case. The median age was 26 years (range, 1 to 43 years). The stem cell source was peripheral blood stem cell (PBSC) in 14 patients, BM in 4 patients, cord blood cell in 1 patient, and both PBSC and BM in 1 patient.
3. Conditioning regimen
The conditioning regimen was TLI on day -1 combined with antithymocyte globulin (ATG; 1.25 mg/kg/day once daily intravenously for 3 days, rabbit type; Pasteur Merieux, Lyon, France) from day -4 to day -2, followed by stem cell infusion (day 0).
The TLI was administered at a dose rate of 22.6 cGy per minute, through anterior and posterior parallel opposed fields, using a 6 MV linear accelerator. The dose of TLI was 750 cGy in 1 fraction on day -1 in 18 of 20 patients. One patient, who previously received TBI with 800 cGy in 4 fractions for 2 days for the conditioning of prior HSCT, received 400 cGy in 1 fraction of TLI reconditioning for the second HSCT. The other patient received 1,000 cGy in 2 fractions of TLI for HSCT with an unrelated matched donor.
Patients were treated with an extended source to skin distance on his or her individual body molds with head fully extended, and supine position for anterior-posterior field and prone position for posterior-anterior field. An akimbo position permitted shielding of the humeral heads. The radiation field was composed of the mantle field and inverted Y field including the spleen. The mantle field extended from the inferior portion of the mandible, and included cervical, supra- and infra-clavicular, mediastinal, and hilar lymph node regions with shielding of the larynx on the anterior-posterior field. The inverted Y field was an infradiaphragmatic field including the spleen, and retroperitoneal, pelvic and inguinal lymph node regions. Radiation fields and the shapes of blocks were verified by portal films in the treatment room prior to treatment.
4. GVHD prophylaxis
The prophylactic regimen for GVHD was the combination of cyclosporine and methotrexate. Cyclosporine was administrated in an intravenous dose of 5 mg/kg/day on day -1. A dose of 3 mg/kg/day was administered intravenously until post-HSCT day 20; then, 5 mg/kg, bid was administered per oral for 2 days from day 21, and then decreased to 3 mg/kg, bid. Methotrexate was administrated intravenously with 10 mg/m2 on days 1, 3, 6, and 11. All patients were treated in rooms with laminar airflow isolation.
5. Definition of engraftment and graft failure
Engraftment was defined as the recovery of the peripheral absolute granulocyte count (ANC) to >0.5 × 109/L on the first of 3 consecutive days, and platelets to >20 × 109/L in the absence of platelet transfusions. Engraftment was also assessed by routine marrow aspirates.
Primary graft failure was considered as ANC of <0.5 × 109/L persisting beyond the 21st day after HSCT, indicating the absence of hematologic recovery. Late graft failure was defined as complete or partial recovery of hematopoiesis of donor origin followed by recurrent pancytopenia (ANC < 0.5 × 109/L without growth factor beyond day 60) with markedly hypocellular BM in the absence of moderate to severe acute GVHD.
6. Statistical analysis
The Kaplan-Meier method was used to estimate the probability of overall survival. Statistical analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Acute and chronic GVHD was evaluated according to the standard criteria [15,16]. Acute GVHD was defined as that which occurred within 100 days after transplantation and chronic GVHD as that which occurred after 100 days.
Results
1. Engraftment and graft failure
Patients were transplanted with a median CD34+ cell dose of 6.0 (range, 1.28 to 14.7) × 106 cells/kg of body weight.
The engraftment failures were found in 6 of 20 patients. Primary graft failure was noted in 2 of 5 patients who received the first HSCT. One patient is alive with the disease, and the other patient who received HSCT from unrelated HLA matched donor died from graft failure, and developed infectious pneumonia and pulmonary hemorrhage at 1 month after HSCT.
Of the 15 patients who received the second HSCT, 4 patients showed late graft failure. Three patients received the third HSCT to be engrafted finally, and 1 patient who could not receive the third HSCT died at 2.3 years after his second HSCT.
Among a total of 20 patients, 17 (85%) patients had sustained engraftment at the last follow-up. The characteristic and outcome of HSCT of 20 patients are shown in Table 2.
2. GVHD
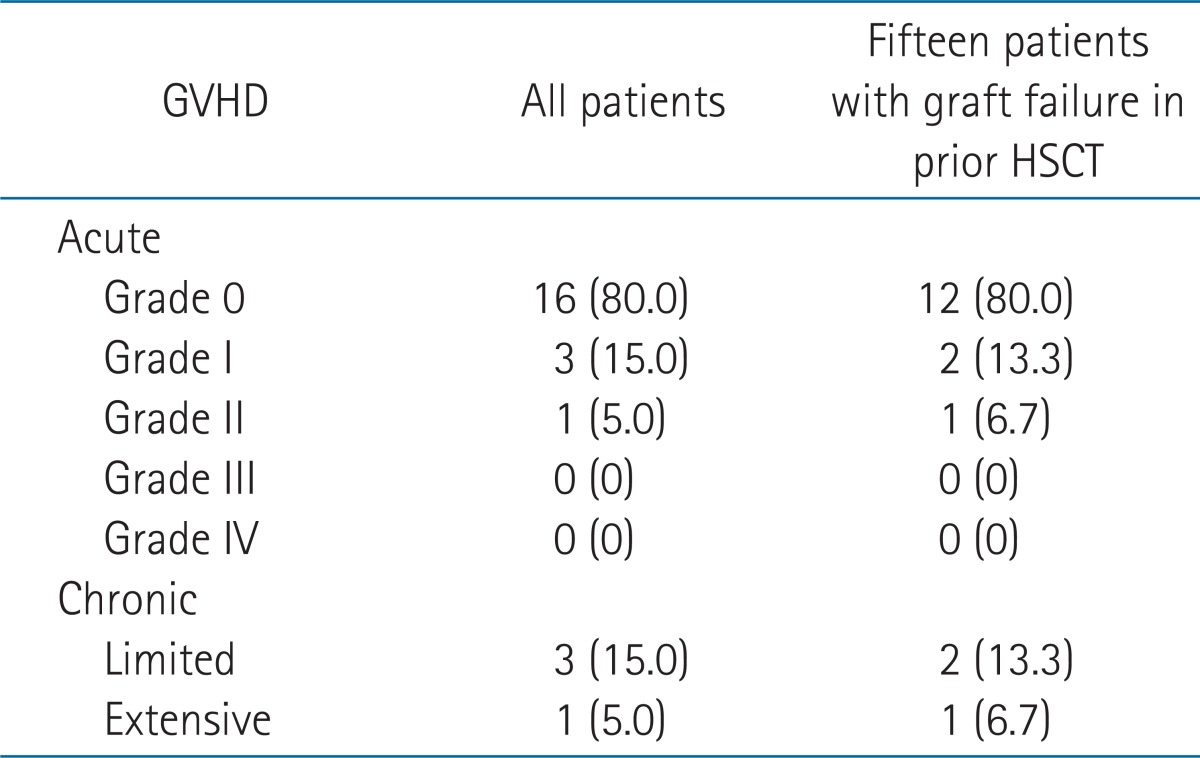
Twelve (60%) patients showed no signs of GVHD. Grade I and II acute GVHD were seen in 15% (3 patients) and 5% (1 patient), respectively. Grade II GVHD of enteritis was treated with systemic prednisone (2-10 mg/kg/day). Once this phenomenon was controlled, the immunosuppression was tapered off according to clinical response. There was no grade III or IV acute GVHD. Chronic GVHD occurred in 4 (20%) of 20 patients. Limited chronic GVHD developed in 3 patients showing mild liver enzyme elevation after 100 days from HSCT. One patient (patient no. 18) showed extensive chronic GVHD with the involvement of oral and intestinal mucosa, and liver after a 6 month follow-up of his second HSCT. He was admitted for 2 weeks and received a steroid pulse therapy. Of the 15 patients who received the second HSCT with TLI based conditioning, acute grade I, II, and chronic GVHD developed in 2 (13.3%), 1 (6.7%), and 3 (20%) patients, respectively. Table 3 summarized the development of GVHD.
3. Other treatment-related complications
Two (10%) patients developed an avascular necrosis of hip joints at 15 and 18 months after HSCT, respectively. One patient showed hypogonadism at 18 months after HSCT. None of the patients have developed a malignancy during the follow-up period.
4. Survival and causes of death
The Kaplan-Meier overall survival curves are shown in Fig. 1. With a median follow-up of 10.8 years (range, 0.1 to 16.4 years), the 5- and 10-year overall survival was 85.0% and 83.1%, respectively, for all patients. Of the patients who received their second HSCT, the 5- and 10-year overall survival was 86.7% and 84.0%, respectively, with a median follow-up of 10.7 years (range, 2.3 to 16.3 years).

Kaplan-Meier estimate of survival for all patients (A), and for the patients who received second hematopoietic stem cell transplantation (HSCT) after previous graft failure (B).
Three deaths were noted at the last follow-up. One patient who received primary HSCT with unrelated HLA matched donor showed primary graft failure, and subsequently died from infectious pneumonia and pulmonary hemorrhage at 1 month after HSCT. Another patient showed fibrothorax and emphysema, which were not related with GVHD, and died from pulmonary complications at 9 years post-HSCT. The other patient showed late graft rejection after the second HSCT and died at 2.3 years after HSCT.
Discussion and Conclusion
TLI was first used as the treatment of all major lymphoid organs in Hodgkin's lymphoma, composed of the mantle field and inverted Y field including the spleen. In the study of TLI-treated patients with Hodgkin's lymphoma, patients were found to have long-lasting alterations in lymphocyte subsets that resulted in an impaired immunological reaction to allogeneic cells but no clinical increase in opportunistic infections [17]. Animal studies later showed that TLI was useful in the induction of specific transplantation tolerance [9,10].
Although the use of irradiation, as part of the conditioning regimen in HSCT, has been reported to increase acute GVHD [12], TLI may be considered to play a role in protecting against acute GVHD; recent investigations have found that the increased percentage of natural killer T cells after TLI could reduce acute GVHD [18]. In the animal experiments, the low incidence of GVHD after TLI was partially related to active suppression of GVHD reactive cells [4]. In addition, as patients with marrow aplasia do not require anti-leukemic doses of conditioning radiotherapy like in other hematologic malignancies, limited field of radiation and de-escalation of radiation dose would be highly desirable. When comparing TLI with TBI, shielding the maximum amount of lungs in TLI can decrease the incidence of interstitial pneumonitis. Moreover, long-term effects on growth, endocrine function, and fertility in children or young patients would be expected to be decreased as well as avoid the development of cataracts [19-21].
In our study, most of the patients were given prolonged immunosuppressive therapy before HSCT, although they had a matched sibling donor. Because the national medical insurance system could not cover HSCT in practice and patients had not received early transplantation. The time elapsed from the diagnosis to the HSCT was significantly longer and thus, patients could not avoid transfusions. Multi-transfused patients were highly sensitized to minor histocompatibility antigens (mHA). The persistence of patient immunocompetent cells, capable of recognizing the mHA difference on donor cells, could result in subsequent graft failures. We used PBSC in 15 patients in order to minimize the risk of graft rejection; however, the shortcoming of PBSC is the increased risk of chronic GVHD [22]. Therefore, TLI, instead of TBI, was selected as the conditioning technique of radiotherapy in order to reduce the GVHD and to enhance the immunosuppressive potential before HSCT. Our patients were followed for a median duration of 10.8 years (range, 0.1 to 16.4 years), which allowed sufficient opportunity to monitor the occurrence of chronic GVHD. We selected a single dose TLI rather than multiple fractions because of the desire to deliver the pretransplant conditioning therapy over a relatively short time period in these pancytopenic patients.
The treatment outcome of SAA has been improved for several decades with survival rate of 60% to 80%. The most recent study reported that HSCT conditioned with cyclophosphamide showed 80% survival rate in young patients who are minimally transfused [2,3]. But the prognosis of high risk patients, who had prior immunosuppressive treatment, and those who were heavily transfused, old age, or had prolonged interval between diagnosis and transplantation, is still poor with the increased risk of graft failure [7,12]. The optimal conditioning regimen for the high risk group has not been well known. Several studies showed favorable results of the reduced rate of graft rejection in heavily transfused patients. Gaziev et al. [19] conducted HSCT for 17 consecutive transfused patients with SAA after conditioning with cyclophosphamide and TLI of 7.5 Gy. They presented no graft failure as well as an actuarial survival rate of 76% on median follow-up for surviving patients of 11 years (range, 0.3 to 14.5 years). They achieved inspiring results; however, only 4 (24%) patients received more than 20 units of transfusions in their study. In our study, all of the patients had history of multiple transfusions with a median of 68 units (range, 10 to 292 units), and 15 (75%) patients received more than 20 units of transfusions.
There were studies that used the combination of TBI and TLI as the conditioning schedule. Novitzky et al. [20] showed encouraging results of radiotherapy-based conditioning regimen for HSCT in SAA. In 17 heavily transfused patients, the conditioning regimen consisted of fractionated TBI of 8 Gy followed by TLI of 6 Gy given in 4 fractions, twice a day, and cyclophosphamide for 2 days. All patients were engrafted. No patients developed GVHD or late graft failure. Inamoto et al. [21] used the conditioning regimen consisting of cyclophosphamide and TLI (7.5 Gy in 2 fraction per a day) for related transplantation, and cyclophosphamide plus TBI (5 Gy in 2 fractions on day -2) and TLI (5 Gy in 2 fractions on day -1) for unrelated transplantation in 49 multi-transfused patients who had undergone previous treatment. ATG was added for 6 of the unrelated transplantation. The overall survival was 81% with a median follow-up of 7 years. The incidence of acute and chronic GVHD was 23% and 29%, respectively. Our results are comparable or superior to the previous results with a high survival rate of 85.0% and 83.1% at 5 and 10 years, respectively, although the patient included had more high risk factors of graft rejection.
The role of TLI in the increased risk of malignancies after HSCT for aplastic anemia is controversial. French study is a frequently cited reference, showing that irradiation is associated with secondary malignancies after HSCT [13]. They reported that 8-year cumulative incidence rate of secondary malignancy was 22% for patients conditioning with cyclophosphamide and 6 Gy of TAI. The secondary solid tumor occurred at a median of 6 years after HSCT. Thereafter, in an analysis of the joint Seattle and aforementioned French group [23], they reassessed the risk factors for developing posttransplant malignancies in 700 patients with aplastic anemia and Fanconi anemia. Multivariate analysis found only two factors (azathioprine therapy and the diagnosis of Fanconi anemia) associated with the development of malignancies, whereas irradiation was a significant factor only if azathioprine was not included in the analysis. But since then, none of the studies have showed the increased risk of secondary malignancy after irradiation based conditioning of HSCT. A large single center study by University of Minnesota [24] used cyclophosphamide and 7.5 Gy single dose of TLI or 13.2 Gy fractionated dose of TBI as the conditioning regimen of HSCT. Of the 150 patients with SAA, one malignancy (B-lymphoproliferative disorder) was observed at a median follow-up of 9.6 years (range, 0.9 to 18.1 years). Gaziev et al. [19] reported no secondary malignancies after cyclophosphamide and 7.5 Gy single dose of TLI for 17 patients. In other studies, which used the combination of TBI and TLI as the conditioning regimen, none of the patients developed secondary malignancy after HSCT with a median follow-up of 3.6 and 7 years, respectively [20,21]. We did not observe secondary malignancy after a median follow-up of 10.8 year after HSCT, corresponding to the above mentioned studies using the limited field of radiation, and further, achieved a low incidence of chronic GVHD. In order to understand the relationship of irradiation based conditioning and secondary malignancy, further study will be necessary because there would be complex possible interactions between various factors, such as etiology of aplastic anemia, age, pretransplant therapy, conditioning regimen, GVHD, and long-term use of immunosuppressive therapy.
In our study, 15 patients received second HSCT after TLI + ATG conditioning. Due to cumulative toxicity of consecutive treatment, the conditioning regimen of second HSCT should be selected carefully. A few studies showing the outcome of second HSCT after graft failure in hematologic disease have been published. The patients' characteristics, conditioning regimen, and stem cell source were heterogeneous in most studies. Moreover, the reports restricted for patients with SAA are rare. According to the report by the Working Party for Severe Aplastic Anemia Registry [25], in 618 patients with SAA treated with the second HSCT, patients receiving irradiation of TLI or TAI in the conditioning regimen prior to their second HSCT showed significantly reduced rate of graft rejection (7% vs. 21%, p = 0.004). The actuarial survival of the whole group was 56% at 12 years. Heinzelmann et al. [26] retrospectively evaluated the efficacy of TLI based reconditioning regimen in 14 patients with various hematologic diseases other than SAA. They all showed a good overall survival rate of 85.7% with no severe toxicity with HSCT, using PBSC at the median follow-up of 22.7 months [26]. In our study, with a median follow-up of 10.7 years for 15 patients with second HSCT (range, 2.3 to 16.3 years), excellent overall survival and engraftment were observed. The 5- and 10-year overall survival rate was 86.7% and 84.0%, respectively. Four (26.7%) out of 15 patients showed late graft failure. Three patients received the third HSCT to be engrafted finally, and 1 patient died at 2.3 years after HSCT. Acute grade I, II, and chronic GVHD was 13.3%, 6.7%, and 20%, which were comparable or lower than previous studies.
In conclusion, our study showed that TLI+ATG conditioning was a feasible immunosuppressive conditioning regimen for HSCT in SAA patients who experienced graft failure from prior HSCT or those who were at a high risk of graft rejection. Adding a single dose of TLI in the conditioning regimen has resulted in a relatively good outcome of engraftment and survival, without increasing the rate of GVHD or second malignancy.
Notes
No potential conflict of interest relevant to this article was reported.