Prognostic value of 18F-fluorodeoxyglucose positron emission tomography, computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with pathologically positive neck lymph node
Article information
Abstract
Purpose
To evaluate the prognostic value of preoperative neck lymph node (LN) assessment with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), computed tomography (CT), and magnetic resonance imaging (MRI) in oral cavity squamous cell carcinoma (OSCC) patients with pathologically positive LN.
Materials and Methods
In total, 47 OSCC patients with pathologically positive LN were retrospectively reviewed with preoperative 18F-FDG PET and CT/MRI. All patients underwent surgical resection, neck dissection and postoperative adjuvant radiotherapy and/or chemotherapy between March 2002 and October 2010. Histologic correlation was performed for findings of 18F-FDG PET and CT/MRI.
Results
Thirty-six (76.6%) of 47 cases were correctly diagnosed with neck LN metastasis by 18F-FDG PET and 32 (68.1%) of 47 cases were correctly diagnosed by CT/MRI. Follow-up ranged from 20 to 114 months (median, 56 months). Clinically negative nodal status evaluated by 18F-FDG PET or CT/MRI revealed a trend toward better clinical outcomes in terms of overall survival, disease-free survival, local recurrence-free survival, regional nodal recurrence-free survival, and distant metastasis-free survival rates even though the trends were not statistically significant. However, there was no impact of neck node standardized uptake value (SUVmax) on clinical outcomes. Notably, SUVmax showed significant correlation with tumor size in LN (p < 0.01, R2 = 0.62). PET and CT/MRI status of LN also had significant correlation with the size of intranodal tumor deposit (p < 0.05, R2 = 0.37 and p < 0.01, R2 = 0.48, respectively).
Conclusion
18F-FDG PET and CT/MRI at the neck LNs might improve risk stratification in OSCC patients with pathologically positive neck LN in this study, even without significant prognostic value of SUVmax.
Introduction
TNM stage, especially N stage, is considered the most important prognostic factor and the most important guide in treatment decisions in patients with oral cavity squamous cell carcinoma (OSCC) [1,2]. Many other prognostic factors such as extracapsular spread (ECS), tumor differentiation, pathologic tumor depth, pathologic resection margins, perineural invasion (PNI), microvascular invasion, and skin invasion have been investigated [3-5]. However, these pathological parameters frequently fail to predict the biological behavior of these tumors. To improve the clinical management of OSCC patients, there is a strong requirement for a more accurate assessment of the malignant properties of the individual lesion.
Computed tomography (CT) or magnetic resonance imaging (MRI) is usually used for preoperative assessment of the primary tumor and cervical status of OSCC. Recently, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) has been widely used in OSCC patients for pretreatment staging while increasing diagnostic accuracy combined with CT or MRI [6,7]. 18F-FDG PET has also contributed to identifying secondary tumors, detecting early recurrences, monitoring the therapeutic outcomes [8,9], and predicting patient survival [10-15]. Prognostic value of 18F-FDG uptake in the primary tumor and neck lymph node has been reported, although the cutoff value of 18F-FDG has still controversy [10-15].
In this study, we aimed to investigate whether pretreatment clinical neck lymph node status assessed by PET and CT/MRI may improve risk stratification in the pathologically node positive OSCC patients. Therefore, identification of reliable prognosticators in this patient group may enable future selection of optimal therapeutic planning.
Materials and Methods
1. Patients
The medical records of 47 consecutive patients meeting the following criteria comprised the study population. Patients had to have histologically proven lymph node positive OSCC without distant metastasis treated by curative resection, including neck lymph node dissections, and adjuvant radio- or chemoradiotherapy between March 2002 and October 2010 at Asan Medical Center; no previous radiation on head and neck area; 18F-FDG PET scan and CT scan or MRI before surgery; and follow-up for survival analysis of at least 20 months. Those patients were identified through a search of the database of radiation oncology department at Asan Medical Center. Patients' staging was performed according to the 2002 American Joint Committee on Cancer (AJCC), 6th edition, staging criteria [1].
2. 18F-FDG PET
All patients fasted for at least 6 hours before the PET examination and received an intravenous injection of 0.2 mCi/kg 18F-FDG after initial preparation. PET acquisition using an axial collimation from the skull base to the proximal femurs was started 60 minutes after injection using an ECAT HR+ scanner (CTI/Siemens, Knoxville, TN, USA). Data were reconstructed into coronal, sagittal, and transverse sections and a 3-dimensional rotating projection. The standardized uptake value (SUV) was calculated from attenuation-corrected images, the amount of injected 18F-FDG, the body weight of each patient, and the cross-calibration factors for 18F-FDG PET and the dose calibrator.
3. CT/MRI
CT of the head and neck was performed with a slice thickness of 3-5 mm. Patients were placed in the supine position, and contrast-enhanced axial images were obtained parallel to the occlusal line from the skull base to the upper chest. In selected patients, direct coronal or coronal reconstruction images were also obtained.
MRI was performed with a 1.5-T or 3.0-T unit using spin-echo technique. Slice thickness was 4-5 mm. T1-weighted images were acquired in the sagittal and axial planes. Axial and coronal T2-weighted fat-suppressed images were also obtained. Thereafter, T1-weighted postgadolinium with fat-suppressed images in axial and coronal projections were obtained sequentially. Positive result was selected, if both CT and MRI were performed and any difference between the result of CT and MRI existed.
4. Image interpretation and analysis
PET results of pathologically positive lymph nodes were scored based on formal interpretations on a 5-point scale as follows: 0, no abnormal uptake; 1, benign; 2, probably benign; 3, probably malignant; and 4, definitely malignant. Scores of 4 to 5 were considered to be positive results for tumor involvement. CT/MRI findings of pathologically positive lymph nodes were also categorized based on formal interpretations into a 2-point scale as follows: 0, negative and 1, positive.
The visual score of PET and CT/MRI were analyzed on neck level by level basis with pathologically positive node. The highest SUVmax were chosen among the SUVmax of pathologically positive nodes, in case of multiple node positive patient.
5. Statistical analysis
Overall survival (OS) was defined as the time interval from date of curative resection until death as a result of any cause or date of last follow-up. Disease-free survival (DFS), local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), distant metastasis-free survival (DMFS) were defined as the time intervals from date of curative resection until any recurrence, local recurrence, regional lymph node recurrence, and distant metastasis, respectively. Survival probabilities were estimated by the method of Kaplan-Meier.
The log-rank test was used to assess the correlation of the clinical outcomes with SUVmax of lymph nodes and clinical positivity of lymph node assessed by PET, CT or MRI. The student t-test and chi-square test were used to evaluate the difference in terms of clincopathologic factors between patients with clinically negative and positive neck node on PET and CT or MRI. A simple regression analysis was used to estimate relations between SUVmax and size of tumor in neck node. In all analyses, values of p < 0.05 were considered to be statistically significant.
Results
1. Patient characteristics
From March 2002 to October 2010, 47 OSCC patients (31 men and 16 women) with pathologically positive node underwent adjuvant radiotherapy after radical surgery at Asan Medical Center. The median follow-up for surviving patients was 56 months (range, 20.1 to 114.3 months). The baseline characteristics of the study participants are shown in Table 1. The neck dissections were ipsilateral in 29 (62%) patients and bilateral in 18 (38%) patients. Only 2 (4%) patients had concurrent chemoradiotherapy with cisplatin, but the others underwent radiotherapy alone. At least 46 Gy of radiation dose delivered to all patients except one who received 23.4 Gy due to poor tolerance.
2. Clinical lymph node findings and survival outcomes
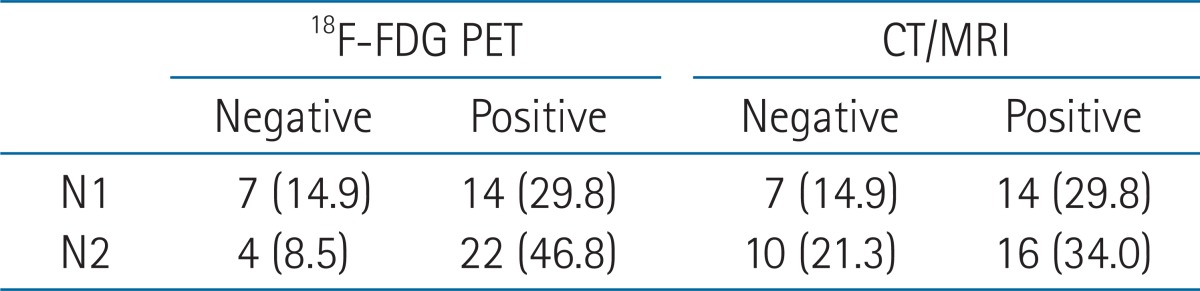
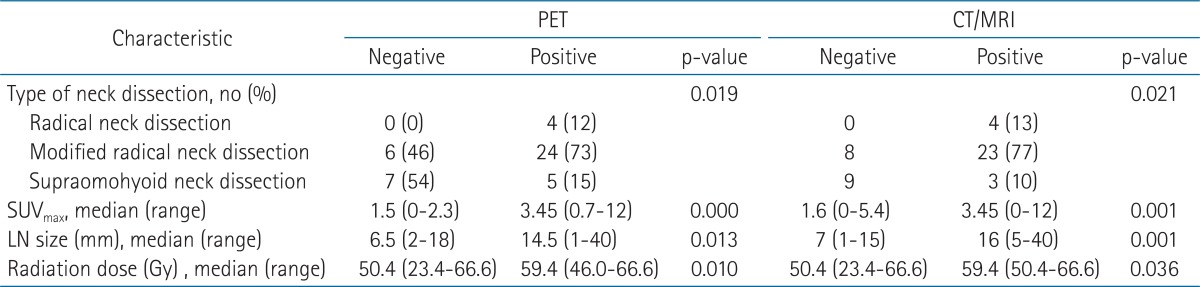
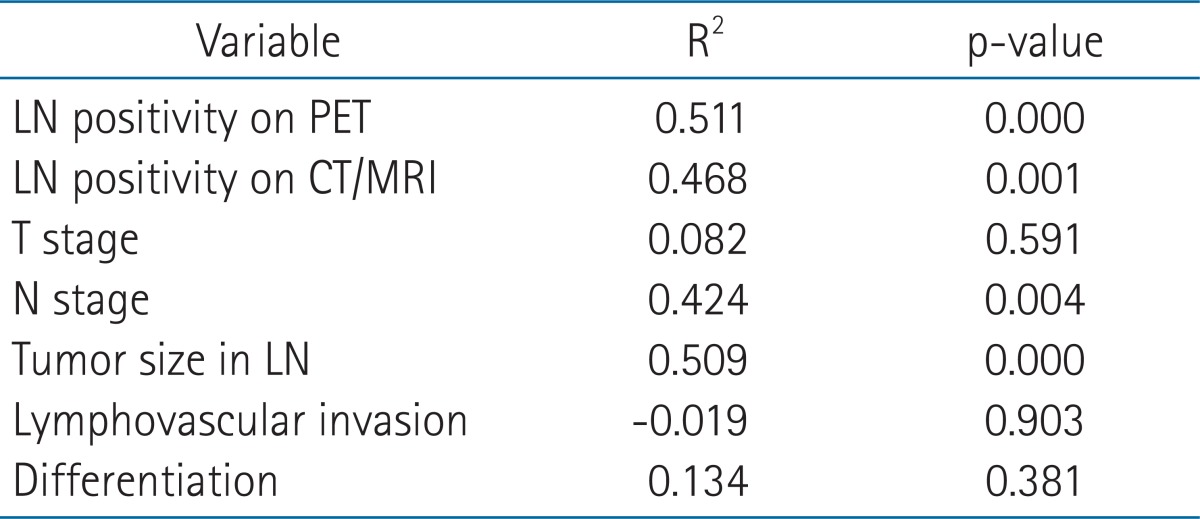
Preoperative lymph node status assessed by PET and CT/MRI was depicted in Table 2. Compared to patients with a PET and CT/MRI-negative neck, those with a PET and CT/MRI-positive neck showed significant differences in type of neck dissection (ND), radiation dose, SUVmax, and tumor size in lymph node among the clinicopathologic factors (i.e., age, gender, tumor location, pT, pN, pStage, lymphovascular invasion, PNI, tumor differentiation, resection margin, extranodal extension, number of metastatic node, tumor size in lymph node, ND, and radiation dose) (Table 3). Notably, SUVmax showed significant correlation with tumor size in lymph node (p < 0.01, R2 = 0.62). PET and CT/MRI status of lymph node also had significant correlation with the size of intranodal tumor deposit (p < 0.05, R2 = 0.37 and p < 0.01, R2 = 0.48, respectively) (Table 4). Clinically negative nodal status evaluated by PET and CT/MRI revealed a trend toward better clinical outcomes in terms of OS (Figs. 1A and 2A), DFS (Figs. 1B and 2B), LRFS (Figs. 1C and 2C), regional nodal recurrence free survival (Figs. 1D and 2D), and DMFS rates (Figs. 1E and 2E) even though the trends were not statistically significant.
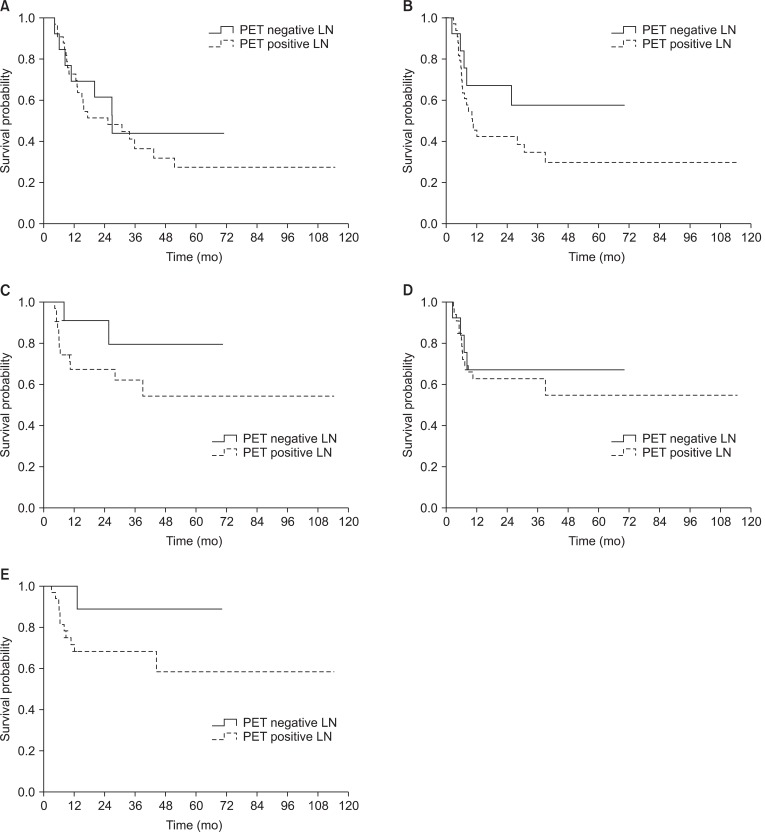
Kaplan-Meier survival analyses according to clinical lymph node (LN) status evaluated by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET); (A) overall survival, (B) disease-free survival, (C) local recurrence-free survival, (D) regional node recurrence-free survival, and (E) distant metastasis-free survival.

Kaplan-Meier survival analyses according to clinical lymph node (LN) status evaluated by computed tomography/magnetic resonance imaging (CT/MRI); (A) overall survival, (B) disease-free survival, (C) local recurrence-free survival, (D) regional node recurrence-free survival, and (E) distant metastasis-free survival.
There was no impact of neck node SUVmax on clinical outcomes. Thirteen SUVmax values from 1 to 10, including median SUVmax value were evaluated by log-rank test to find a so-called best cutoff, none of them showed statistically significant difference in any survival outcomes.
3. Univariate analyses
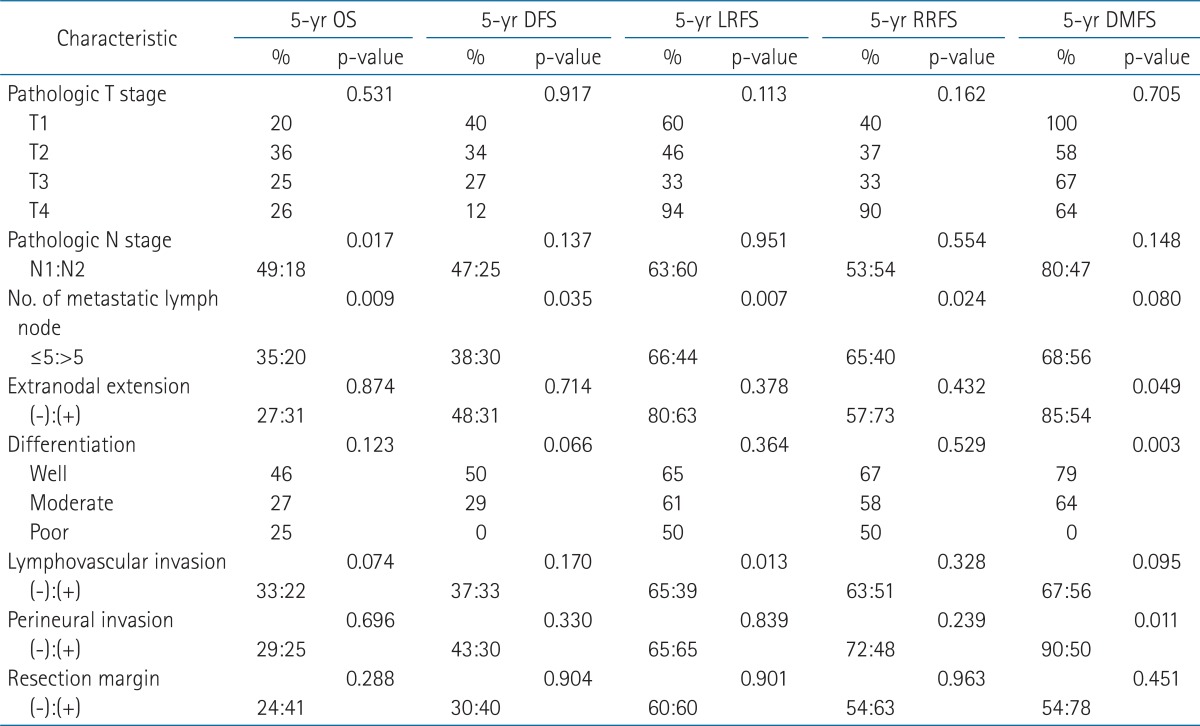
The results of univariate analyses of 5-year local and neck control, DMFS, DFS, and OS are shown in Table 5. Patients with pN1 stage had better 5-year OS rate than those with pN2 stage. Patients who had 5 or less metastatic lymph nodes showed significantly better OS, DFS, LRFS, and RRFS rates.
Discussion and Conclusion
Our principal finding in this study was that clinical lymph node status on PET and CT/MRI might improve the risk stratification in pathologically lymph node positive OSCC patients, even without significant prognostic value of SUVmax.
Many investigators have suggested that high FDG uptake at primary tumor, which was obtained by SUVmax, is associated with both poor response to and survival after several therapies in patients with various cancers, including OSCC [9-12,16-27]. Mostly, the prognostic value of a FDG uptake may be independent of the tumor stage. Nonetheless, a variety of standard cutoff value of SUVmax and way of choose the standard cutoff value is suggested in each study. It has hampered the reliability of those investigations on the prognostic value of SUVmax.
The presence of cervical metastases is the most important prognosticator for outcome in OSCC patients [2]. However, little has been reported about the prognostic significance of clinical findings in the neck lymph nodes on either PET or CT/MRI in patients with OSCC. A SUVmax of 5.7 at lymph node was presented as an independent prognosticator for 5-year neck cancer control and survival rates in OSCC patients with pathologically positive lymph nodes [13]. Liao et al. [14] showed PET findings of the neck lymph nodes may improve risk stratification beyond that of traditional risk factors in OSCC.
The present study is the first one to investigate the prognostic value of pretreatment lymph node status on both PET and CT/MRI in pathologically node positive OSCC patients. Patients with PET-negative neck revealed the trend toward better LRFS, RRFS, DMFS, and DFS rates than those with PET-positive neck. Survival graphs also showed better LRFS, DMFS, DFS, and OS rates in patients with negative neck node on CT/MRI than those with CT/MRI-positive neck. These results were statistically insignificant. However, small number of patients could be one of the reasons for statistical insignificance. Therefore, neck status on PET and CT/MRI had the potential to improve risk stratification in node positive OSCC patients and were worthy of further investigations.
Pathological and biological mechanisms underlying correlation between SUVmax and survival have not yet been fully investigated. High SUVmax of primary tumor exhibited greater tumor thickness and depth of invasion than those with low SUVmax [11,12,28]. Clinical significance of correlation between SUVmax and Glut-1, one of the 13 glucose transporters, expression varied upon the different type of cancers [28-32]. Bcl-2, anti-apoptotic protein, expressed at high level in OSCC patients with high SUVmax [28]. We found the patients with high SUVmax at lymph node and PET, CT/MRI-positive neck had significantly larger size of intranodal tumor deposit diameter than those with low SUVmax and clinically negative neck. However, it couldn't fully explain the survival difference because the size of intranodal tumor deposit was not a significant prognosticator at univariate analyses. The biologic and pathologic mechanisms of correlation between clinical node status and survival should be investigated in the future. Two important limitations of our study were the small number of patients and its retrospective design.
In conclusion, pretreatment lymph node status evaluated by PET and CT/MRI may have the capacity to improve risk stratification for clinical outcomes in patients with pathologic node positive OSCC. It should be verified through the further prospective, large clinical trial.
Notes
No potential conflict of interest relevant to this article was reported.