Prognostic implications of tumor volume response and COX-2 expression change during radiotherapy in cervical cancer patients
Article information
Abstract
Purpose
The relationship between treatment outcomes, alteration of the expression of biological markers, and tumor volume response during radiotherapy (RT) in patients with uterine cervical cancer was analyzed.
Materials and Methods
Twenty patients with cervical squamous cell carcinoma received definitive RT with (n = 17) or without (n = 3) concurrent chemotherapy. Tumor volumes were measured by three serial magnetic resonance imaging scans at pre-, mid-, and post-RT. Two serial punch biopsies were performed at pre- and mid-RT, and immunohistochemical staining for cyclooxygenase (COX)-2 and epidermal growth factor receptor was performed. The median follow-up duration was 60 months.
Results
The median tumor volume response at mid-RT (V2R) was 0.396 (range, 0.136 to 0.983). At mid-RT, an interval increase in the distribution of immunoreactivity for COX-2 was observed in 8 patients, and 6 of them showed poor mid-RT tumor volume response (V2R ≥ 0.4). Four (20%) patients experienced disease progression after 10 to 12 months (median, 11 months). All 4 patients had poor mid-RT tumor volume response (p = 0.0867) and 3 of them had an interval increase in COX-2 expression. Overall survival (OS) and progression-free survival (PFS) decreased in patients with V2R ≥ 0.4 (p = 0.0291 for both). An interval increase in COX-2 expression at mid-RT was also associated with a decreased survival (p = 0.1878 and 0.1845 for OS and PFS, respectively).
Conclusion
Poor tumor volume response and an interval increase in COX-2 expression at mid-RT decreased survival outcomes in patients with uterine cervical cancer.
Introduction
Concurrent chemoradiotherapy (CCRT) is the treatment of choice for advanced uterine cervical cancer [1-3]. Treatment outcomes have been found to be influenced by the International Federation of Gynecology and Obstetrics (FIGO) stage, pelvic or para-aortic lymph node metastasis, and tumor size measured at pre-, mid-, or post-radiotherapy (RT) [4-7]. In particular, tumor volume regression rate at mid-RT is a predictor of local control rate and disease-free survival (DFS) after RT or CCRT [6,7]. In addition, several biological markers such as cyclooxygenase (COX)-2, epidermal growth factor receptor (EGFR), vascular endothelial growth factor, and inducible nitric oxide synthase (iNOS) have been shown to be associated with tumor control and survival after RT [8-13]. Synchronous coexpression of COX-2 and EGFR or COX-2 and iNOS has been shown to be a prognostic factor for predicting poor survival [12,13].
In a previous study, we attempted to define the relationship between the tumor volume response and alteration of the expression of biological markers during RT. We reported that the tumor volume response during RT was negatively affected by the coexpression of COX-2 and EGFR in cervical cancer patients [14]. Although it was marginally significant, poor tumor volume response during RT was associated with an interval increase in the distribution of immunoreactivity for COX-2 at mid-RT compared with that of pre-RT. However, the clinical outcomes according to the expression of the biological markers and tumor volume response to RT could not be analyzed. In this report, we analyzed the relationship between treatment outcomes and alteration of the expression of biological markers during RT in cervical cancer patients who were treated with RT.
Materials and Methods
1. Patients and treatment
From March 2005 to November 2006, we prospectively enrolled twenty consecutive patients with cervical squamous cell carcinoma. After approval of the Institutional Review Board, informed consent was obtained from all patients. The characteristics of patients are summarized in Table 1. A combination of whole pelvis RT and high-dose-rate (HDR)-intracavitary radiation (ICR) by a remote after-loading system using Ir-192 was delivered. The prescribed dose to the whole pelvis was 50.4 Gy in 28 fractions using the 4-field box technique of 15 MV photons, and the dose of HDR-ICR to point A was 24 Gy in 6 fractions which was delivered twice a week. Seventeen patients received CCRT, while the remaining three patients who had FIGO stage IB or IIA tumors, which were smaller than 3 cm, received RT alone. The chemotherapeutic regimens were 6 cycles of weekly cisplatin (30 mg/m2) during RT for 11 patients, and 2 or 3 cycles of 5-fluorouracil (1,000 mg/m2) plus cisplatin (60 mg/m2) every 3-4 weeks during RT for the remaining 6 patients. The median follow-up duration, calculated from the start of RT, was 60 months (range, 14 to 82 months).
2. Tumor volume measurement using magnetic resonance imaging (MRI)
The time schedule of MRI examinations and the method of tumor volume measurement have been previously described [7,14]. In brief, MRIs were taken at the start of the RT (pre-RT), at the fourth week of RT (mid-RT) and 1 month after RT finished (post-RT). Three-dimensional tumor volumes were calculated by summation of all the areas of the tumor in each slice outlined on T2-weighted images and multiplied by the slice interval. The pre-RT, mid-RT, and post-RT tumor volumes were defined as V1, V2, and V3, respectively. Tumor volume responses at mid-RT (V2R) or post-RT (V3R) were obtained by dividing V2 or V3 by V1, respectively.
3. Immunohistochemical staining for biological markers
At pre-RT and mid-RT, punch biopsies, with the intent to obtain an adequate sample of tumor tissue, were performed. The presence of tumor was confirmed on the hematoxylin and eosin-stained slides, and then immunohistochemical (IHC) staining for COX-2 and EGFR was subsequently performed. The details of IHC staining have been previously described [14]. The intensity and the distribution of the immunoreactivity were examined by two experienced pathologists. The intensity was divided into negative, weak, moderate, and strong staining. The specimen was classified as positive when the distribution of moderate to strong immunoreactivity accounted for more than 10% of the sample. If the IHC-stained slide was available at mid-RT, the change of the distribution of moderate to strong immunoreactivity was compared with that obtained at pre-RT.
4. Clinical endpoints and statistical analysis
The endpoints of this study were overall survival (OS) and progression-free survival (PFS) according to the parameters of tumor volume response and the expression of biological markers. OS was defined as the time from the start of RT to death from any cause, and PFS was defined as the time from the start of RT to disease progression, relapse, or death. Disease progression was defined as a 25% or greater increase of tumor volume or recurrent tumor during the follow-up period. Kaplan-Meier analysis and log-rank test were used for estimation and comparison of the survival outcomes. To examine the correlations between the parameters of tumor volume and the expression of the biological markers, Fisher's exact test was used. We used the SAS ver. 9.1.3 (SAS Institute Inc., Cary, NC, USA) for statistical analysis. A probability value of less than 0.05 was considered statistically significant.
Results
1. Tumor volume and immunoreactivity for biological markers
The median tumor volumes at pre-RT and mid-RT were 20.9 cm3 (range, 1.7 to 132.3 cm3) and 9.3 cm3 (range, 0.4 to 73.5 cm3), respectively. The median tumor volume response at mid-RT (V2R) was 0.396 (range, 0.136 to 0.983). At post-RT, 5 (25%) patients had residual tumor that ranged from 1.7 cm3 to 6.6 cm3, while the remaining 15 patients had complete regression of tumor.
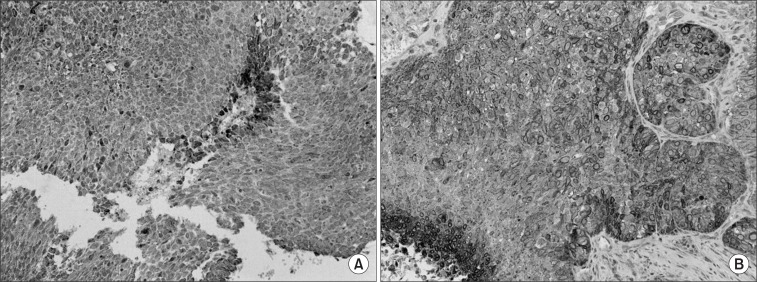
Fourteen (70%) and eleven (55%) patients were positive for COX-2 and EGFR by IHC at pre-RT, respectively. Positive immunoreactivity for both the biological markers was observed in 8 patients. Because of tumor necrosis by radiation or sampling error, there were 12 slides IHC-stained for COX-2 and EGFR available at mid-RT. Of these, an interval increase in the distribution of immunoreactivity was observed in 8 (67%) and 6 (50%) cases for COX-2 and EGFR, respectively (Fig. 1).
2. Relationship between tumor volume response and the expression of biological markers
Nine out of the 10 patients whose V2R was greater than 0.4 showed positive immunoreactivity for COX-2 at pre-RT (p = 0.1409). In 8 patients who showed positive immunoreactivity for both biological markers, poor volume response at mid-RT (V2R ≥ 0.4) was observed in 6 (75%) patients (p = 0.1698). Among the 8 patients who had an interval increase in the distribution of immunoreactivity for COX-2 at mid-RT, 6 (75%) patients showed poor volume response at mid-RT, although it was not statistically significant (p = 0.2222).
3. Treatment outcomes
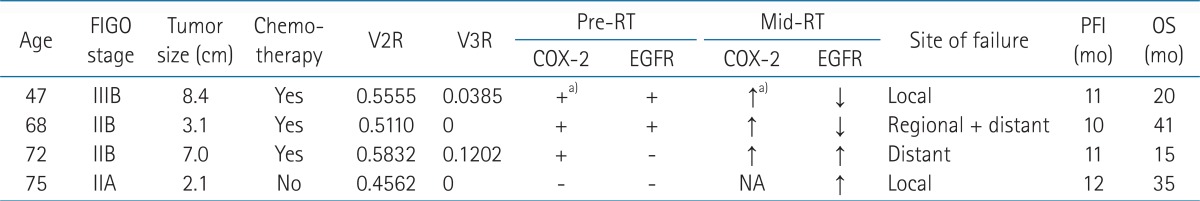
During the follow-up period, 4 (20%) patients experienced disease progression after 10 to 12 months (median, 11 months) of a progression-free interval. Local tumor progression occurred in 2 patients, regional recurrence in 1 patient, and distant metastasis in 1 patient. As shown in Table 2, all 4 patients had poor mid-RT tumor volume response (p = 0.0867) and 3 of them had an interval increase in immunoreactivity for COX-2 (p = 0.4909), although the small number of patients limited statistical significance. Two of them had residual tumor at post-RT.
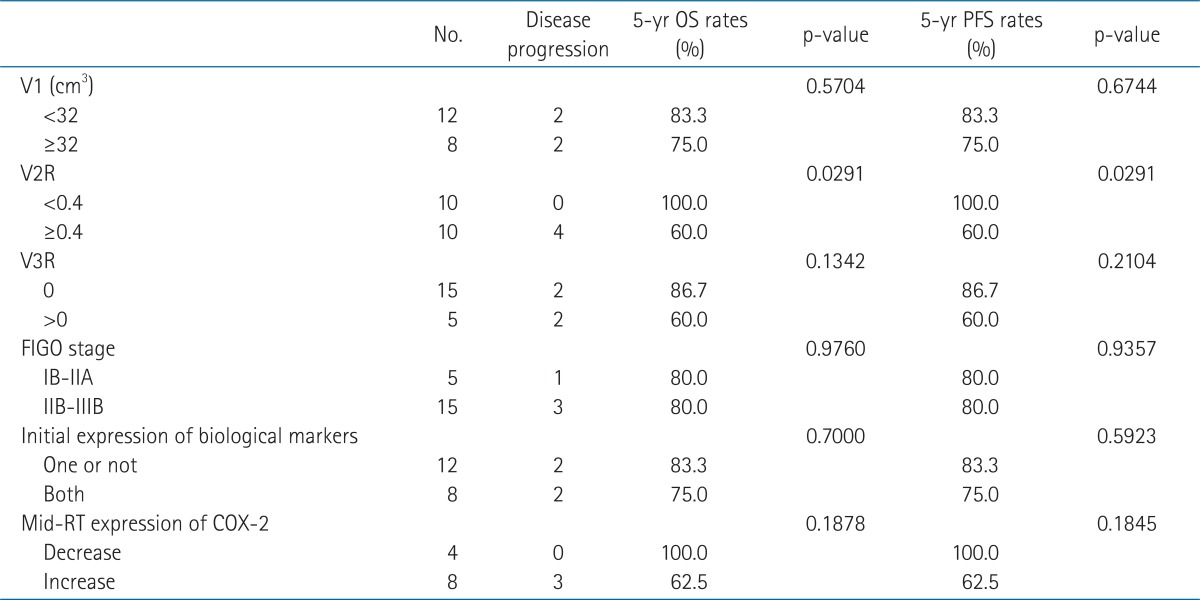
The 5-year OS and PFS rates of all patients were both 80%. The survival rates of the patients whose V2R greater than 0.4 were both 60%, which was significantly poorer than that of the patients with V2R less than 0.4 (100%, p = 0.0291 for both OS and PFS). The survival rates of the 8 patients with an interval increase in immunoreactivity for COX-2 were both 62.5%, while that of the 4 patients with an interval decrease were 100% (p = 0.1878 and 0.1845 for OS and PFS, respectively). PFS according to V2R or mid-RT expression of COX-2 is shown in Fig. 2. The coexpression of COX-2 and EGFR at pre-RT, initial stage, and initial tumor volume were not associated with survival outcomes by log-rank test (Table 3).
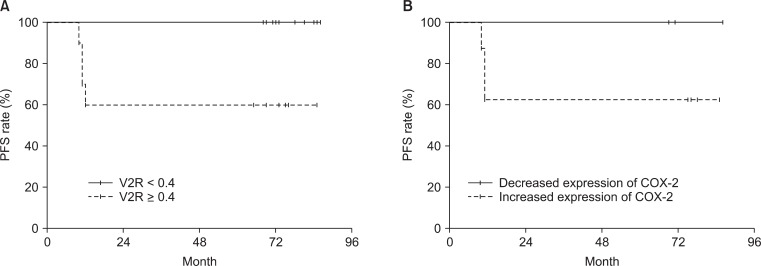
Progression-free survival (PFS) according to tumor volume response (A) and interval change of cyclooxygenase (COX)-2 expression (B) at mid-radiotherapy. Diminished survival was observed in patients with poor tumor volume response (p = 0.0291) or an interval increase of COX-2 expression (p = 0.1845). V2R, percentage of residual tumor volume at mid-radiotherapy.
Discussion and Conclusion
The conclusion of our previous report was that poor tumor volume response at mid-RT was associated with coexpression of COX-2 and EGFR at pre-RT [14]. After a median 60 months of follow-up, the survival outcomes were negatively affected by poor mid-RT tumor volume response and an interval increase in immunoreactivity for COX-2 at mid-RT. These findings suggest that volumetric or biologic responses evaluated at mid-RT could be predictors of survival outcomes in patients with cervical cancer.
Mid-RT tumor volume regression has been reported as a predictor of local control and DFS [6,7]. Local control rate differed by residual tumor volume (<20% vs. ≥20%) at 45 to 50 Gy (84% vs. 22%, p < 0.0001) [6]. DFS was also affected by mid-RT residual tumor (63% vs. 20%, p = 0.0005). In the present study, all patients who experienced disease progression showed poor mid-RT tumor volume response. In addition, biologic response evaluated by IHC at mid-RT may be another prognostic factor for survival outcomes. Out of the 8 patients who had an interval increase of immunoreactivity for COX-2, disease progression was observed in 3 patients, while there was no progressive disease among the 4 patients with a decreased immunoreactivity. Although the difference was not statistically significant, mid-RT biologic response could be suggested as having a prognostic implication.
COX-2 is associated with carcinogenesis, tumor proliferation and progression [15]. There was a significant negative correlation between the apoptotic index of biopsy specimens from cervical cancer patients and pre-RT expression of COX-2, which suggested that COX-2 inhibits radiation-induced apoptosis [16]. It is also associated with decreased survival [8,9]. The overexpression of COX-2 decreased the 5-year OS rate from 75% to 35% in patients who received definitive RT for FIGO stage IB-IIIB cervical cancer [8]. Inversely, inhibition of COX-2 demonstrated an antitumor effect and enhanced response to radiation [17,18]. Based on these observations, a multi-institutional phase II trial of celecoxib, a COX-2 selective inhibitor, combined with CCRT was performed [19,20]. Unfortunately, locoregional control was problematic and the incidence of acute toxicities was high. The authors concluded that more active biologically targeted therapies need to be identified [20].
The combination of a COX-2 inhibitor and an EGFR tyrosine kinase inhibitor was then proposed as an alternative [21-23]. Activation of the EGFR signaling pathway increases transcription of COX-2 via p38 mitogen-activated protein kinase [24,25]. Decreased survival outcomes by expression of EGFR have been reported in several studies [10,11]. In addition, the coexpression of COX-2 and EGFR significantly reduced DFS in patients who underwent CCRT with FIGO stage IB cervical squamous cell carcinoma, although the correlation between the levels of EGFR and COX-2 immunoreactivity was small [12]. Our previous and present studies demonstrated that coexpression of COX-2 and EGFR was negatively associated mid-RT tumor volume response, but it did not affect survival outcomes. Survival outcomes were influenced by an increased COX-2 immunoreactivity and tumor volume response at mid-RT. EGFR expression was also not associated with either volume response or survival outcomes.
Nitric oxide could be another target. It increases the activity of the COX-2 pathway via cellular cyclic guanosine monophosphate (GMP) [26,27]. Positive correlation between expression of iNOS and COX-2 (Spearman correlation coefficient, 0.49; p < 0.01) was observed by Chen et al. [13]. Along with advanced stage (III to IV) and large tumor size (≥4 cm), the coexpression of iNOS and COX-2 was an independent prognostic factor influencing DFS (p < 0.01). The antitumor effect of NOS inhibitor on head and neck squamous cell carcinoma has been demonstrated in xenograft model [28]. Inhibition of NOS has an antitumor effect on COX-2-overexpressing carcinoma cells [29]. In addition, a combination of selective iNOS and COX-2 inhibitors showed a protective effect in colon carcinogenesis [30]. Thus, the combination might be a novel biologically targeted approach.
Individualized approaches based on radiologic, biologic, and metabolic information are needed for treatment of uterine cervical cancer patients to improve treatment outcomes and to reduce treatment-related toxicities. Recently, metabolic tumor response determined by positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) has been shown to be predictive of treatment response and survival outcomes in patients with cervical cancer [31,32]. The use of FDG-PET is not confined to prediction of treatment outcomes. ICR as an essential component of curative RT can be delivered using 3-dimensional image-guided brachytherapy [33,34]. In addition to MRI, PET with or without fusion with computed tomography images can be used in 3-dimensional brachytherapy planning [35,36]. Radiation dose escalation using intensity-modulated RT, consolidation chemotherapy after CCRT, and introduction of new targeted agents are examples of individualized approaches [37-40].
Although this is a novel study examining the correlation between the alteration of biological markers during RT and treatment outcomes, there are some limitations as including a small number of patients and small successful sampling during RT due to tumor necrosis. In conclusion, survival outcomes of patients with uterine cervical squamous cell carcinoma were negatively affected by poor volume response and an interval increase of COX-2 expression at mid-RT. Individualized treatment approaches including biologically-targeted therapy should be considered to overcome expected poor clinical outcomes in patients with poor tumor volume response during RT or CCRT.
Acknowledgments
This work was supported by a Samsung Biomedical Research Institute grant (SBRI C-A6-414-1).
Notes
No potential conflict of interest relevant to this article was reported.