Introduction
Individuals predisposed to keloid scars have a tendency to produce excessive fibrous tissue in response to skin trauma. This tissue ultimately extends beyond the wound, hyalinizes, and rarely regresses spontaneously. Not only are such keloids unsightly, they also frequently cause itching and pain [1].
While steroid injections, topical patches and/or creams [2-4], and laser treatment [5,6] have all been shown to be successful treatment modalities, long-term recurrence rates for all of these treatments are still considerable. Accordingly, the preferred treatment for keloids is the excision of the excess tissue, followed by a procedure to prevent fibroblast proliferation, the main cause of recurrences. Because recurrence rates after excision alone are very high [7], surgery is not recommended as monotherapy. Instead, postoperative radiotherapy is a more effective and less painful treatment option associated with fewer side effects [8-10].
The women with keloid often develop protruding scars after their first Cesarean delivery. These keloids are typically associated with uncomfortable symptoms, such as itching and/or pain, in addition to their undesirable formation and discoloration. In this study, in cases of repeat Cesarean sections, the keloids were completely excised and postoperative radiotherapy was considered. We analyzed the clinical results, side effects, and resulting cosmetic appearances of postoperative radiotherapy for the treatment of abdominal keloid scars secondary to Cesarean section.
When radiotherapy is used to treat abdominal keloid scars in women of reproductive age, ovarian radiation exposure must be considered. In this study, doses of radiation to the ovaries were measured in a solid phantom under fixed clinical conditions in order to confirm ovarian safety.
Materials and Methods
In total, 26 women were recruited as subjects between January 2009 and June 2010. Women who have scheduled a repeated Cesarean section and had keloids on their previous abdominal operation scar are taken radiotherapy only in case of last delivery (Table 1). The median age for this cohort was 34 years, with a range of 30 to 44 years. Of these individuals, 65% reported symptomatic itching and/or pain.
Linear accelerators (Clinac IX; Varian Medical Systems, Palo Alto, CA, USA) were used to produce a 6-MeV electron beam. The total radiation dose was either 12 or 15 Gy and was divided into three fractions; radiotherapy was initiated within 24 hours of the Cesarean section. In order to deliver the maximum dose of radiation to the skin surface, a 1-cm-thick tissue equivalent bolus material was used. A customized lead cutout was used for radiation protection.
After completing treatment the subjects underwent serial physical examinations to evaluate both the cosmetic results and side effects at one month, six months, one year, and then once every year. During follow-up period, we asked the patients about self satisfactory level graded into four such as excellent, good, sufficient, and unsatisfactory.
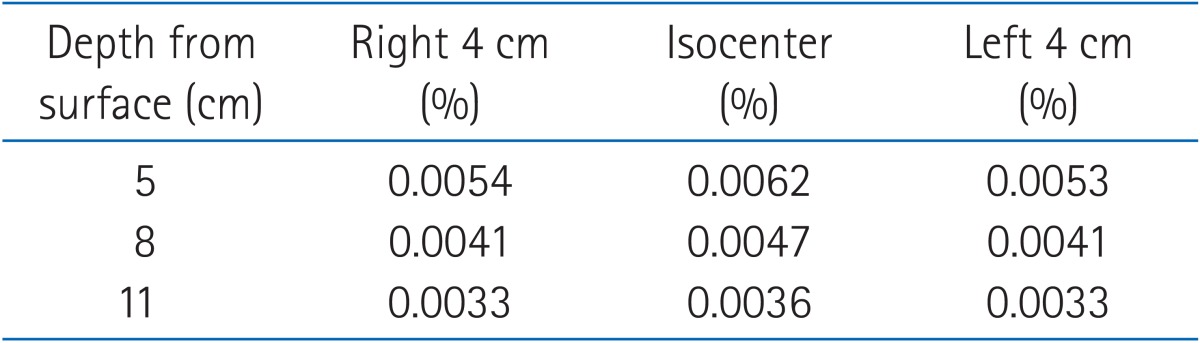
To assess the radiation exposure to the ovaries, pelvic magnetic resonance images from 20 randomly-selected women in our hospital were reviewed, with mean transcutaneous ovarian depths ranging from 5 to 11.4 cm (mean, 8.3 cm). The mean distance between ovaries was approximately 8 cm. Based on these result, doses were measured at depths of 5, 8, and 11 cm and ±4 cm lateral to the isocenter at each level.
The measurement system was set up with the same specifications as were used in the treatment phase, with a source to surface distance of 100 cm. All measurements were performed using a solid plate phantom (SP34; Wellhofer/IBA, Schwarzenbruck, Germany) with an ionization chamber (CC13; Wellhofer/IBA) and a width of 30 × 30 cm2. An electron applicator measuring 20 × 20 cm was also installed using the same lead cutout created for post-Cesarean section scar treatment. Additionally, as with the actual treatment sessions, a tissue equivalent bolus with a thickness of 1 cm was used.
Results
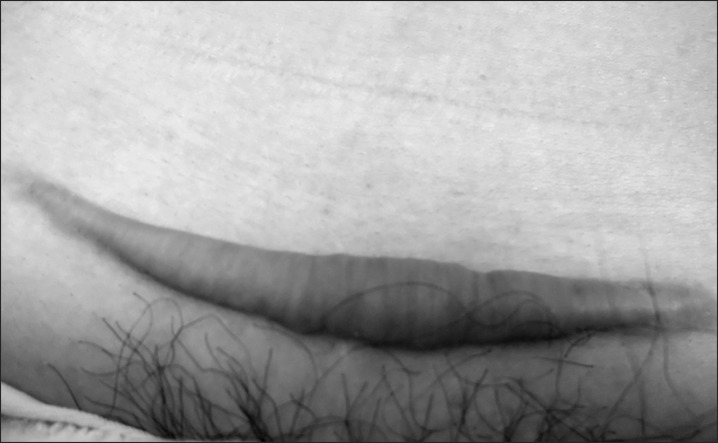
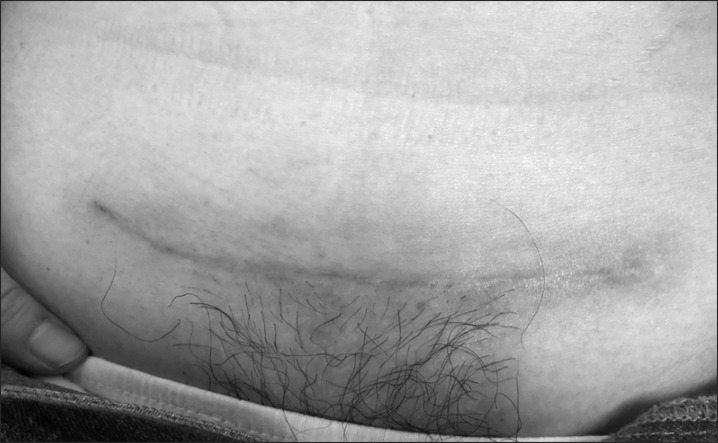
Follow-up times ranged from 19 to 36 months (median, 27 months). Twenty patients (77%) achieving a complete response. An additional six patients (23%) experienced areas of focal elevation in the center of the operative scar, measuring 0.5 to 2 cm in length. No acute side effects, such as erythema or wound dehiscence, were observed, although five patients did report areas of mild hyperpigmentation. Similarly, no late complications, such as new telangiectasia or atrophy, were observed. None of patients have complained of itching or pricking pain after treatment. Self-assessment results of the treatment were rated as good to excellent in most patients (96%), with Figs. 1 and 2 showing pre- and post-treatment pictures of the abdominal operative scars.
Table 2 demonstrates the measured doses of cutaneously administered radiation to the designated ovarian depths, ranging from 0.0033% to 0.0062%. In patients treated with total doses of radiation ranging between 12 and 15 Gy, the corresponding ovarian exposure was estimated to be between 0.0396 and 0.093 cGy.
Discussion and Conclusion
Currently, the best therapeutic modality for keloids appears to be a multistep approach comprised of surgery plus any one of several adjuvants, including intralesional steroids, silicone gel sheeting, pressure, or radiotherapy, among others. Specifically, most patients treated with monotherapies such as silicone gel sheeting or ointments after their first Cesarean section still experienced symptomatic keloid scars. Consequently, in this study, postoperative radiotherapy was used to prevent repeat Cesarean section scarring after the original keloid was completely excised during delivery.
After surgical excision of the primary keloid, orthovoltage-based radiotherapy has been demonstrated as a good method for preventing further relapse [11], with only 14.6% of patients requiring additional adjuvant treatment. Typical dose-fractionation schemes range from 9 to 16 Gy in 3 to 4 fractions [12]. Dose response relationship is still controversial. Some reports suggested higher local control rate with higher radiation dose [9,11]. We prescribed 12 or 15 Gy in 3 fractions and the local control rate and adverse effects showed satisfactory outcomes.
Bischof et al. [13] achieved excellent local control (85%) and good to excellent self-satisfaction ratings (62%) with radiation therapy, reporting that subjective assessments did not always fully correspond to clinical examination or incidence of recurrence. In our study, despite experiencing some degree of local recurrence, many subjects nonetheless remained satisfied with their treatment results.
In general, postoperative radiotherapy is well tolerated and has few side effects. In their study of post-treatment scarring that was clinically and objectively tested using the Vancouver scar scale and a durometer, Akita et al. [14] concluded that the combined regimen of surgical excision and radiation therapy is effective for improving scar quality. Moreover, in a separate paper describing their 20-year experience with postoperative radiotherapy for the treatment of keloids, Caccialanza et al. [15] reported a complete remission rate of 90% and a five-year relapse-free rate of 87%. In our results, no instances of full relapse occurred, and partial recurrences were observed in only 23% of subjects, many of which developed after some episode of focal irritation or infection after completion of radiotherapy. After analyzing the therapeutic outcomes associated with this modality, Ogawa et al. [16] concluded that anatomic sites with increased stretch tension were associated with statistically higher recurrence rates. Accordingly, these authors suggested that sites at increased risk for recurrence should be treated with increased doses of radiation and more aggressive post-treatment self-management.
Nonetheless, long-term follow-up of at least two years duration is key to predicting successful treatment [17]. Therefore, longer periods of follow-up are required to better evaluate the differences in treatment results and cosmesis between the 12 and 15 Gy regimens.
The human ovary is extremely sensitive to radiotherapy. In fact, by extrapolating data generated from the model and graphically depicting the risk of developing acute ovarian failure (AOF, defined as failure within five years of diagnosis) after stratification according to total ovarian radiation exposure, Wallace et al. [18,19] suggested that the dose required to destroy 50% of immature oocytes (lethal dose 50) is less than 2 Gy. Although this mathematical model has not yet been validated through additional clinical studies, Wo and Viswanathan [20] suggested that 6 Gy yielded a low risk to individuals between 25 and 30 years of age using above mathematical model. Schmidt et al. [21] also evaluated ovarian doses in postoperative keloid treatment with calibrated by ion chamber and TLD. They reported that ovaries at a depth of 10 cm in the central beam received a dose of between < 1 mGy in electron therapy. In our study, the total ovarian dose was less than 0.093 cGy, confirming the safety of postoperative radiotherapy for abdominal keloid scarring in women of reproductive age.
In conclusion, postoperative radiotherapy is an effective treatment for keloid secondary to repeated Cesarean sections, providing excellent local control with less side effects. However, additional studies are needed to further assess the long-term response to therapy and the late side effects associated with this treatment.