Method of tumor volume evaluation using magnetic resonance imaging for outcome prediction in cervical cancer treated with concurrent chemotherapy and radiotherapy
Article information
Abstract
Purpose
To evaluate the patterns of tumor shape and to compare tumor volume derived from simple diameter-based ellipsoid measurement with that derived from tracing the entire tumor contour using region of interest (ROI)-based 3D volumetry with respect to the prediction outcome in cervical cancer patients treated with concurrent chemotherapy and radiotherapy.
Materials and Methods
Magnetic resonance imaging was performed in 98 patients with cervical cancer (stage IB-IIIB). The tumor shape was classified into two categories: ellipsoid and non-ellipsoid shape. ROI-based volumetry was derived from each magnetic resonance slice on the work station. For the diameter-based surrogate "ellipsoid volume," the three orthogonal diameters were measured to calculate volume as an ellipsoid.
Results
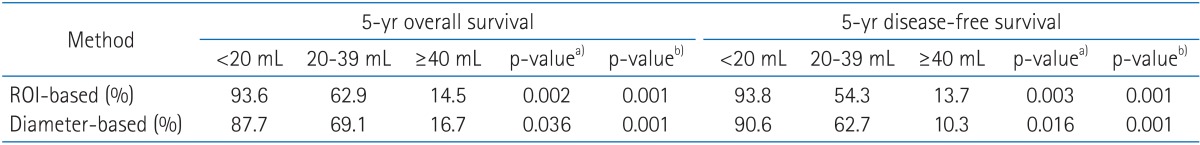
The more than half of tumor (55.1%) had a non-ellipsoid configuration. The predictions for outcome were consistent between two volume groups, with overall survival of 93.6% and 87.7% for small tumor (<20 mL), 62.9% and 69.1% for intermediate-size tumor (20-39 mL), and 14.5% and 16.7% for large tumors (≥40 mL) using ROI and diameter based measurement, respectively. Disease-free survival was 93.8% and 90.6% for small tumor, 54.3% and 62.7% for intermediate-size tumor, and 13.7% and 10.3% for large tumor using ROI and diameter based method, respectively. Differences in outcome between size groups were statistically significant, and the differences in outcome predicted by the tumor volume by two different methods.
Conclusion
Our data suggested that large numbers of cervical cancers are not ellipsoid. However, simple diameter-based tumor volume measurement appears to be useful in comparison with ROI-based volumetry for predicting outcome in cervical cancer patients.
Introduction
The size and volume of the primary tumor are well-established prognostic factors and predictors of the radiation therapy outcome for cervical cancer patients [1-14]. With the advent of magnetic resonance imaging (MRI), it has become possible to estimate the size of the primary tumor more accurately than was prior possible by clinical palpation or computer tomography (CT) in inoperable patient [15-17]. Tumor size and local invasion in the surgical specimen correlates well with the corresponding diameter measured in T2-weighted MRI [18]. MRI provides a non-invasive method for tumor size and volume evaluation in cervical cancer who are planned to be treated by radiotherapy [19].
For clinical practice, the diameter-based tumor size measurement has been the standard method to assess tumor size and volume [9,11,12]. This method is used under the assumption that the configuration of the tumor closely approximates an ellipsoid shape and employed simple diameter measurement of three orthogonal diameters that can be performed on film hard copies [20]. Diameter-based measurement is an established method that is easy and fast to perform in a busy clinical practice. The disadvantage is that the measurement may be less accurate because tumor shape of cervical cancer is irregular configurations that deviate from idealized ellipsoid volume [9]. With the advancement and availability of commercially available computer software for quantitative analysis in cross-sectional imaging, the entire tumor, regardless of its shape, can be identified and traced as a region of interest (ROI) on each imaging slice, and the 3-dimensional (3D) ROI-based quantitative measurement of tumor volume can be performed in clinical setting [5,8-10,13].
Despite the increasing importance of imaging-based tumor volume in cancer treatment, the optimal method for adequate volume measurement for tumor has not been defined and remains controversial [9,10,21,22].
The purpose of this study was to evaluate the patterns and distribution of tumor shape and to compare tumor volume derived from simple diameter-based ellipsoid measurement with that derived from tracing the entire tumor contour morphology using more complex ROI-based 3D tumor volumetry with respect to the prediction outcome in cervical cancer patients treated with concurrent chemotherapy and radiotherapy.
Materials and Methods
1. Patients population
Ninety-eight patients with newly diagnosed cervical cancer, referred for definitive radiation therapy between 1999 and 2003 were registered. Ethical approval for the study was obtained from the institutional ethics committee. The patients population included the International Federation of Gynecology and Obstetrics (FIGO) stage IB2 (n = 8), IIA (n =10), IIB (n = 64), IIIA (n = 5), and IIIB (n = 11). There were 86 patients with squamous cell carcinoma, 7 with adenocarcinoma, and 5 with adenosquamous cell carcinoma. Ages ranged from 30 to 65 years (median, 55 years).
Pretreatment evaluations included a history and physical examination, tumor biopsy, complete blood count, serum chemistries, chest radiograph, pelvis MRI and intravenous pyelogram (IVP) or abdominopelvic computer tomography (CT). Cystoscopy and/or proctoscopy were performed when clinically indicated. Each patient's disease was staged according to the FIGO classification system. Evaluations during radiotherapy consisted of a physical examination, including a pelvic examination. Other studies such as complete blood count and serum chemistries performed weekly during radiation therapy in selected patients when indicated.
2. Treatment policy
Patients were treated by concurrent chemotherapy and radiation therapy with a curative intent. Patients received both external beam radiation therapy (EBRT) and intracavitary brachytherapy (ICRT). The EBRT was given with 10 MV photons in all patients. The EBRT was administered using an isocentric technique via a four field box technique. The treatment field was set to extend 3 cm beyond the known extent of disease and to encompass the iliac and lower common iliac lymph nodes. Patients were treated in the prone position with a full bladder. A dose of 45.0 Gy was administered to the whole pelvis at 1.8 Gy per fraction. ICRT consisted of 30 Gy in 6 fractions using high dose rate (HDR) brachytherapy, given twice weekly. Parametrial or nodal boost, if required, consisted of 9-10 Gy in 1.8-2 Gy fractions given in between the ICRT fractions using EBRT. In addition, cisplatin was given intravenously once a week at a dose of 40 mg per square meter of body surface area, with the total dose not to exceed 70 mg per week, during the course of their external beam radiotherapy. A maximum of six doses of cisplatin was given.
3. MRI protocol
Immediately before the start of radiotherapy, MRI was performed on all patients. MRI examinations were obtained on a 1.5 T scanner (Gyroscan; Philips Medical Systems, Eindhoven, Netherlands) using the system's body coil. All patients were scanned supine with abdominal compression to minimize respiratory motion artifacts. Imaging included sagittal 5-mm (4-mm thickness with 1-mm gap) conventional fast spin echo T2-weighted image (effective echo time [TE], 104; repetition time [TR], 4,000; echo-train length, 10; number of excitations [NEX], 2); and axial 7-mm (5 mm thickness with 2 mm gap) T2-weighted and T1-weighted images (TE, 16; TR, 600; NEX, 2).
4. Tumor volume measurement and tumor configuration analysis
The tumor was defined as an abnormal area with intermediate to high signal intensity on T2-weighted images with respect to the surrounding cervical stroma and uterus. The reviewer reviewed all serial magnetic resonance (MR) studies of each of patients and qualitatively classified the tumor configuration into two categories: ellipsoid and non-ellipsoid shape. The ellipsoid category was defined as smooth configuration without lobulations, closely approximating a round or oval shape. The non-ellipsoid category was defined as an irregular configuration with lobulations.
The tumor volume was assessed in each MR examination independently by ROI-based volumetry and by diameter-based measurements. For the ROI-based measurement, the entire tumor region identified and traced on the MR workstation on all T2-weighted sagittal imaging slices throughout the tumor (Fig. 1). A 3D ROI-based volume was calculated by the summation of all tumor areas in each slice and multiplication by the slice profile (4-mm slice thickness plus 1-mm gap). The diameter-based calculation was computed by measuring the largest tumor diameter in each orthogonal measurement plane (Fig. 2). The craniocaudal diameter (dcc) along the long axis of the endometrial cavity was measured on the sagittal images; the anteroposterior diameter (dap, orthogonal to the craniocaudal diameter) was measured on the sagittal images; and the largest lateral diameter (dl) was measured on the axial images. Diameter-based measurements were computed as an ellipsoid (V = dcc × dap × dl × Π / 6) to calculate diameter-based volume (V).

Region of interest volumetry. Sagittal T2-weighted image in a 58-year-old woman with stage IIB cervical cancer showing the magnetic resonance (MR) image with the largest tumor cross-section. The black line indicates the tracing of the region of interest for the tumor volume measurement. The areas which are traced on each sagittal T2-weight image are summed to calculate tumor volume (V). The tumor area (A) in each MR slice, V = 0.6 (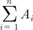 ). n, number of slice; i, individual slice number.
). n, number of slice; i, individual slice number.

Diameter-based measurement. Sagittal T2-weighted image in the same patients with in Fig. 1. The craniocaudal diameter (dcc) and anteroposterior diameter (dap) are shown. The lateral diameter (dl) is determined in the axial plane. Diameter-based tumor volume (V) is then computed with the ellipsoid formula (V = dcc × dl × dap × Π / 6).
5. Follow-up
The patients received follow-up by the radiation oncologist every 1-2 months for the first 6 months, then every 3 months for the first 2 years, and then every 4 months thereafter. Follow-up evaluations included a history and physical examination, pelvic examination, Pap smear, complete blood count, and serum chemistries. Chest X-rays were obtained yearly. CT scans of the abdomen and pelvis, MR images, or bone scans were obtained if clinically indicated.
6. Statistical analysis
The correlation between the tumor volumes derived from the diameter-based and ROI-based measurement methods was performed using linear regression analysis. The correlation coefficients between the two measurement methods were analyzed with respect to the distribution of the tumor configurations. Tumor volume was correlated with local control, disease-free survival (DFS) and overall survival (OS) rates using the Kaplan-Meier life table analysis for the data sets from each measurement method. Differences between patient groups were evaluated with the log-rank test. Local failure was defined as tumor recurrence during the follow-up period or persistent/progressive tumor within the pelvis. For the computation of DFS, death due to causes other than cervical cancer was considered a censored observation. For OS, death of any cause was scored as an event. Tumor size parameters were identified for each, ROI-based volume and diameter-based volume, by stepwise correlation of size thresholds with the outcome endpoints. The tumor size was computed in increments of 20 mL tumor volume, and each increment was correlated with the outcome parameters (local control, DFS, and OS). The tumor volume thresholds of 0-19 mL, 20-39 mL, and ≥40 mL were thus derived.
Results
Ninety-eight patients were investigated for the analysis. The mean follow-up time for patients was 38 months (range, 4 to 84 months).
1. Qualitative analysis of tumor configuration
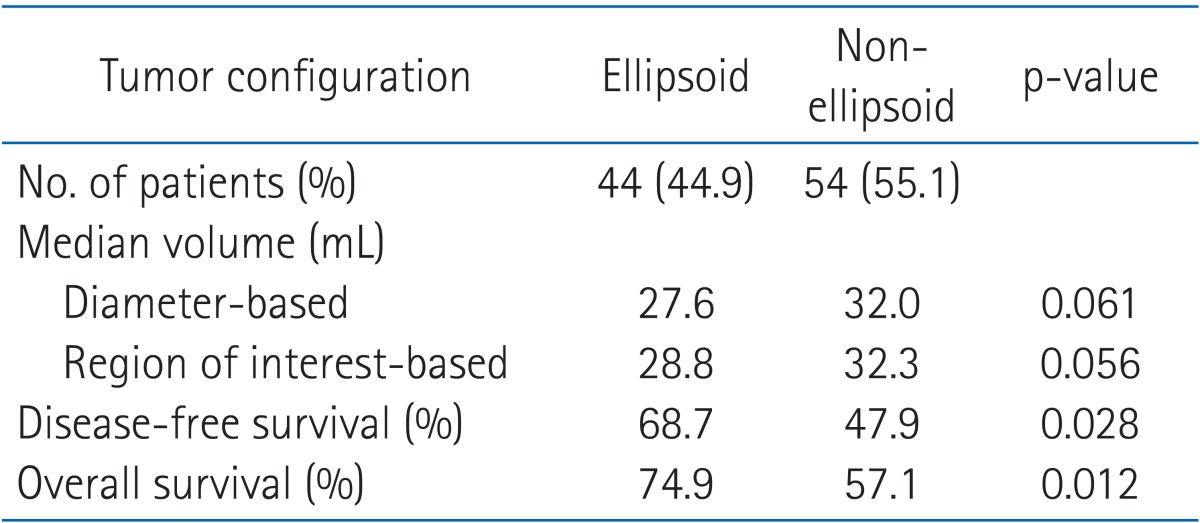
The configuration patterns of the tumors were not equally distributed: 44.9% ellipsoid and 55.1% non-ellipsoid. Compared with the ellipsoid configuration, the median tumor volume of non-ellipsoid configuration by the diameter-based and ROI-based methods were slightly larger (32.0 mL vs. 27.6 mL in diameter-based and 32.3 mL vs. 28.8 mL in ROI-based method). Non-ellipsoid configuration had a significantly worse OS (57.1%) and DFS (47.9%) compared with OS (74.9%, p = 0.012) and DFS (68.7%, p = 0.028) with ellipsoid configuration (Table 1).
2. Analysis of tumor volume measurement methods
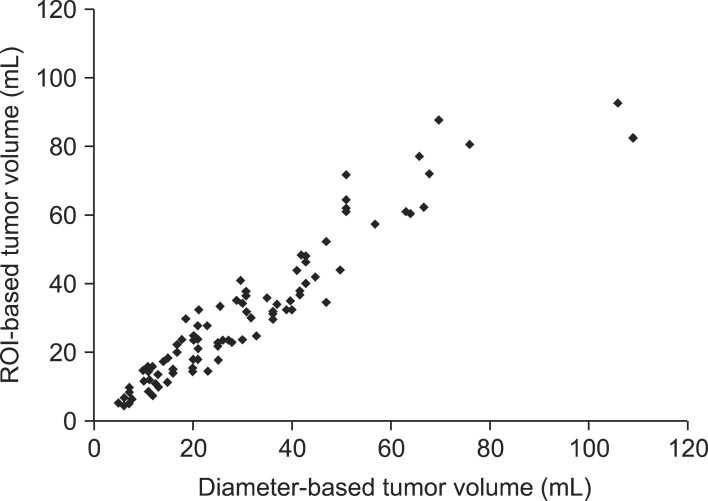
Fig. 3 summarizes the correlation of the tumor volumes derived from diameter-based and ROI-based measurements. The median tumor volume measured by the diameter-based method was similar with the ROI-based volume (26.8 mL vs. 25.2 mL). The diameter-based tumor volume measurement had the strong correlation with ROI-based measurement (γ = 0.91, p = 0.001).
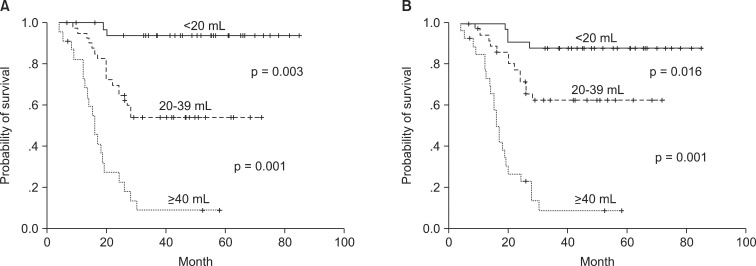
The correlation of tumor volume with the outcome endpoints showed similar results between the simple diameter-based method and the ROI volumetry method. The predictions for OS, DFS, and local control were consistent between two volume groups, with OS rate of 93.6% and 87.7% for small tumor (<20 mL), 62.9% and 69.1% for intermediate-size tumor (20-39 mL), and 14.5% and 16.7% for large tumors (≥40 mL) using ROI and diameter based measurement, respectively (Table 2, Fig. 4). DFS rate were 93.8% and 90.6% for small tumor, 54.3% and 62.7% for intermediate-size tumor, and 13.7% and 10.3% for large tumor using ROI and diameter based method, respectively (Fig. 5). Differences in outcome between each volume groups from different methods were statistically significant.
Comparison of outcome correlation based on tumor volume between region of interest (ROI)-based and diameter-based method

Overall survival curves of the groups divided by tumor volume with 3-dimensional region of interest measurement (A) and diameter-based measurement (B).
Discussion and Conclusion
Radiation therapy has played essential role in treatment of cervical cancer. Several randomized control trials have shown the significant prolongation of both OS and DFS in groups treated by concurrent chemotherapy and radiotherapy [23-25]. Accurate staging in cervical carcinoma is essential in therapeutic decision-making, determining prognosis, and comparing the results of different treatment modalities in these inoperable patients. Recently, primary tumor size has been well established in a large number of studies as an important independent predictor to survival in inoperable cervical cancer [1-14]. Therefore, the appropriate method and its accuracy for tumor size measurement to provide significant information is critical for cervical cancer treatment. Classically, the size measurement of the primary tumor has been largely based on physical pelvic examination. Although this is convenient and cost-effective, the accuracy of tumor size measurement is unsatisfactory [1]. Clinical palpation is also a subjective method and has significant interobserver variability [26-28]. More recent studies have shown that the three-dimensional quantitative imaging-based method of tumor size measurement using MRI is highly accurate in determining actual tumor size and extent to surrounding tissue [2,3,15,16,29-37]. MRI has been recognized as an important imaging modality for the management of cervical cancer because of its multiplanar capability and distinct tissue contrast, particularly between tumor and surrounding normal tissue [9].
Narayan et al. [12] demonstrated that, tumor volume measured by diameter-based method in advanced cervical cancer provide important prognostic information over and above that provided by FIGO stage, clinical tumor diameter, histology, and age. Other authors have suggested that pre-treatment ROI-based tumor volume was significant prognostic factor for patients with invasive cervical carcinoma [5,8,11,14,38]. However, the ideal method for the accurate measurement of primary tumor volume remains controversial [9,10,21]. A 3D ROI-based tumor volumetry has been documented as the most accurate noninvasive tumor volume measurement in cervical cancer based on a classic surgical correlation study of 3D ROI imaging measurements with 3D ROI measurement of histologic giant tissue sections of the pathologic specimens [9,15]. But imaging-based tumor volume assessment, the tumor volume by diameter-based method have been traditionally estimated and reported to be equivalent to the more complex 3D ROI volumetry that delineates the entire tumor region three dimensionally [23,33,39].
The method of diameter-based measurement is the simple, fast and more practical in busy clinical practice. However, the estimation of tumor volume by the diameter-based method relies on the assumption that the configuration of the tumor is ellipsoid. It does not consider irregularities in tumor border and shape, which can be accounted for by three-dimensional ROI-based volumetry method. On the other hand, the ROI-based quantitative volume measurement is more complex and time-consuming method than the diameter-based measurement because it requires image analysis by tracing the entire tumor contour on multiple MRI slices [9]. Regardless of its shape, the ROI-based volumetry measurement includes all tumor components identified on all images throughout the lesion. Therefore, tumor-specific deviations from the ideal ellipsoid shape do not compromise its accuracy [10].
Our results show that for the prediction of treatment outcome in cervical cancer, method of diameter-based measurement of pretreatment tumor volume is adequate and there in no need for ROI volumetry. Similarly, Mayr et al. [9] reported that for the pre-treatment measurement both the diameter-based method and ROI volumetry in cervical carcinoma treated with radiation therapy alone provided similar predictive accuracy, particularly for patients with small (<40 cm3) and large (≥100 cm3) pre-radiotherapy tumor size. However, the predictive value of tumor volume in our results measured by either method has limitation. In our data, tumor volume was obtained from only pretreatment MRI. Some reported that diameter-based and ROI-based measurement correlated well before radiation therapy but not during radiation therapy [10]. Tumor regression rate obtained during mid-radiotherapy, which was appreciated by 3D ROI based volumetry, had been reported to be the best outcome prediction factor for local control and DFS [9].
Our results show that more than half of the cervical cancers (55.1%) did not have a shape closely approximating the ellipsoid configuration. Compared with the ellipsoid configuration, the median tumor volume of non-ellipsoid configuration by the diameter-based and ROI-based methods were slightly larger than that of ellipsoid configuration. Therefore, non-ellipsoid configuration might have a significantly worse survival compared with ellipsoid configuration. Similarly, Mayr et al. [10] reported that most cervical cancers are not ellipsoid in shape before treatment and that tumor configuration becomes increasingly irregular and non-ellipsoid during and after therapy because of nonconcentric tumor shrinkage. They also suggested that ROI-based volumetry, which can optimally measure irregular volumes, may provide better response assessment during treatment than diameter-based measurement.
Our study does not have histologic validation of the imaging findings. This is a challenge in the imaging of unrespectable cervical cancer and in many other unrespectable cancers. But a European study by Burghardt et al. [15] reported that the volumes obtained by MRI correlated well (r = 0.983) with those obtained by histomorphometric analysis of the surgical specimens in cervical cancer (stage I and IIB tumors, which are treated surgically in Europe).
The patient population was accrued over a relatively long time. At the time of study, new pulse sequences with improved spatial resolution and volume acquisition techniques as well as phase array coils were not available. If this study were repeated today, the results may be different because of the improved tumor delineation by newer advanced imaging techniques.
Our overall patient numbers are not large enough at this time to allow multivariate analysis to systematically assess tumor volume parameters with respect to other prognostic factors. Our data will need further confirmation with larger patient numbers.
In conclusion, our limited data suggested that large numbers of cervical cancers are not ellipsoid in configuration. In spite of that, simple diameter-based tumor volume measurement appears to be useful in comparison with ROI-based 3D volumetry for predicting outcome in cervical cancer patient treated with concurrent chemotherapy and radiation therapy.
Notes
No potential conflict of interest relevant to this article was reported.