 |
 |
AbstractPurposeTo determine whether the maximum standardized uptake value (SUV) of [18F] fluorodeoxyglucose uptake by positron emission tomography (FDG PET) ratio of lymph node to primary tumor (mSUVR) could be a prognostic factor for node positive non-small cell lung cancer (NSCLC) patients treated with definitive radiotherapy (RT).
Materials and MethodsA total of 68 NSCLC T1-4, N1-3, M0 patients underwent FDG PET before RT. Optimal cutoff values of mSUVR were chosen based on overall survival (OS). Independent prognosticators were identified by Cox regression analysis.
ResultsThe most significant cutoff value for mSUVR was 0.9 with respect to OS. Two-year OS was 17% for patients with mSUVR > 0.9 and 49% for those with mSUVR ≤ 0.9 (p = 0.01). In a multivariate analysis, including age, performance status, stage, use of chemotherapy, and mSUVR, only performance status (p = 0.05) and mSUVR > 0.9 (p = 0.05) were significant predictors of OS. Two-year OS for patients with both good performance (Eastern Cooperative Oncology Group [ECOG] ≤ 1) and mSUVR ≤ 0.9 was significantly better than that for patients with either poor performance (ECOG > 1) or mSUVR > 0.9, 23% (71% vs. 23%, p = 0.04).
IntroductionLung cancer is the leading cause of cancer death, having caused an estimated 1.18 million deaths worldwide in 2002 [1]. Although current treatment strategies do not cure most patients, there have been improvements in the outcome over time. Definitive radiotherapy (RT) is the main treatment modality for medically inoperable or locoregionally advanced non-small-cell lung cancer (NSCLC). Nevertheless, in the cases of advanced disease, more than 90% of patients die from the disease [2]; therefore, there is a need to identify a subset of NSCLC patients with longer life expectancies. Clinico-pathologic features of NSCLC have been studied, but are not commonly used in prediction and stratification of patients' prognosis, with the exception of stage and performance status [3,4].
During the last decade, [18F]fluorodeoxyglucose positron emission tomography (FDG PET), which is based on differences in glucose metabolism between tumor and normal tissue, has been incorporated into the cancer management process, for staging [5-8], estimation of treatment response [9-14], and delineation of RT targets [15] for NSCLC. In addition, a review of 13 studies found that the maximum standardized uptake value (maxSUV) of the primary tumor has a prognostic value in NSCLC [16]. In spite of some debates with regard to the role of FDG PET as a prognostic factor [17,18], it is widely accepted that maxSUV on FDG PET is a significant prognostic factor. However, maxSUV of primary tumor has some limitation because tumors usually demonstrate heterogeneity in biological and metabolic characteristics with progression or metastasis. Especially, the characteristics of metastatic cancer cells are crucial because they are more closely correlated with prognosis, after treatment of primary lesions. Therefore, biological and metabolic characteristics of metastatic lesions are recommended to be considered in prognostication.
Although the histology of mediastinal lymph nodes is crucial in the treatment strategy, FDG PET is becoming more useful in mediastinal staging of the lung cancer. In one study, an unique concept was proposed that a ratio between the maxSUV of the tumor and the lymph nodes predicts nodal pathology in patients with NSCLC [19] . Moreover a generation of the metabolic ratio may take into account the different techniques and circumstances used in a scanning of patients. At this finding we hypothesized that the metabolic ratio of NSCLC could be a better prognostic predictor of lung cancer, than metabolic activity of the tumor or the lymph node. To verify this hypothesis, two parameters for metabolic activity of lymph nodes were adopted for analysis; maxSUV of lymph node and ratio of maxSUV between primary lesion and lymph node.
Materials and Methods1. PatientsThe medical records of 70 consecutive NSCLC patients who were treated with definitive RT at our institution from October 2004 to December 2008 were retrospectively reviewed. Patients with suspicious lymph node disease and absence of M1a and M1b metastatic disease formed the study group. Patients with recurrent tumors were excluded. Pretreatment evaluation consisted of history taking and physical examination, contrast-enhanced chest computed tomography (CT), FDG PET/PET-CT scan, and pulmonary function test. No patients were staged with mediastinoscopy, and lymph nodes were considered suspicious for metastatic disease if the short axis diameter was larger than 1 cm on chest CT or metabolically active. There were 7 patients with a lymph node sized 1 cm or smaller, and the maxSUV of these nodes ranged 3 to 22. At final, 68 patients were included in the final analysis, because 2 patients were excluded from analysis due to uncertain histology. All patients were staged using the American Joint Committee on Cancer (6th edition) TNM system.
2. Positron emission tomographyThe time intervals between PET or PET-CT and initial treatment were less than 8 weeks. Before FDG PET scans, patients fasted for at least 6 hours. Using dedicated PET or PET-CT scanners (ECAT EXACT47 and Biograph 40, Siemens, Malvern, PA, USA; GEMINI, Philips Medical Systems, Cleveland, OH, USA), images were acquired from the skull base to the upper thigh 60 minutes after 5.18 MBq/kg (0.14 mCi/kg) of FDG administration. Most of patients (64 of 68) underwent PET-CT and only 4 patients underwent PET only. Images were reconstructed using iterative reconstruction algorithms (3D row-action maximum-likelihood algorithm and ordered subset expectation maximization). Region of interests (ROIs) were drawn over main masses on trans-axial images, and standard uptake values (SUVs) were calculated using the following equation: ROI tissue activity concentration × lean body mass / injected dose. Maximal uptakes of primary tumor and lymph node were presented as maxSUVP and maxSUVLN, respectively. The maxSUV of a given subject in the present study represents the highest maxSUV of the whole subject lesions. All the images were reviewed by two experienced nuclear medicine physicians. After the maxSUVP and maxSUVLN were obtained, the ratio between them was calculated as maxSUVLN to maxSUVP, which was coined as mSUVR.
3. TreatmentAfter the FDG PET study and a definitive diagnosis, 43 patients (63%) were treated with concurrent chemoradiotherapy, 21 patients (31%) were treated with neoadjuvant chemotherapy before RT, 16 patients (24%) were treated with chemotherapy after RT, and 14 patients (21%) were treated with RT only due to old age (n = 12) and comorbidity (n = 2). Platinum-based multi-drug regimens were administered to most patients.
All patients were treated with involved-field RT, once a day, 5 days a week, and a total dose of 60 to 66 Gy with CT planning. The gross tumor volume (GTV) consisted of all known sites of disease defined by CT with the visual aid of FDG PET or PET-CT scan data, without inclusion of elective nodal targets. The clinical target volume was determined from the GTV with an automatic expansion of 1 cm. The planning target volume included the physiologic motion and respiratory movement, which ranged from 1 to 2 cm. Critical normal structures were also contoured. Almost all patients were treated with conventional fractionation (1.8 to 2.0 Gy) without a planned treatment break, and only 3 patients were treated with hypo-fractionation (4 Gy) for poor performance and comorbidity.
4. Statistical analysisSurvival status was evaluated in November 2012. Distant metastasis was defined as an appearance of disease at other than the primary site or regional lymph nodes. Metastases to any organs, such as the brain, bone, adrenal glands, contralateral or ipsilateral lung (in a different lobe) were considered distant metastasis, and nodal stations other than the mediastinal and the intrapulmonary nodal chain as non-regional stations. All events observed by not only radiological but clinical or physical examinations were considered significant. Distant metastasis-free survival (DMFS) and overall survival (OS) rates were calculated by use of the product-limit method of Kaplan-Meier. All estimates were calculated from the date of pathologic diagnosis until the defined event, if any, or until the last contact or death. Patients were censored at the time of last follow-up. The log-rank test was used for assessment of the equality of the survivor across groups. The Cox proportional hazards model was used for multivariate analysis to assess the effect of patients' characteristics and other prognostic factors of significance, including the maxSUV of the primary tumor and lymph node and the mSUVR, on the end points. All tests were two tailed, with p < 0.05 considered statistically significant. SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
ResultsMedian follow-up from the date of diagnosis in all patients was 20 months (range, 4.2 to 84.6 months) with a median follow-up among survivors of 42 months (range, 14.7 to 84.6 months). No patient was lost to follow-up; median OS for the entire patient population was 21.3 months.
The median maxSUV of the primary tumor and the lymph node was 10.3 (range, 2.1 to 26.3) and 6.6 (range, 0 to 21.9), respectively. The short axis diameter of five lymph nodes with maxSUV < 3 ranged from 1.5 to 2.6 cm. Seven patients (10%) had metabolically active but normal sized nodes. The median mSUVR was 0.63 (range, 0 to 1). We performed sensitivity analyses in order to determine the mSUVR value that best differentiated patients into subgroups of prognosis, and the value of 0.9 was the most discriminative cutoff mSUVR value for OS (Fig. 1).
The median OS of 50 patients (74%) with mSUVR ≤ 0.9 was 26 months (95% confidence interval [CI], 19 to 32 months). The median OS of 18 patients (26%) with mSUVR > 0.9 was 11 months (95% CI, 6 to 16 months). The median DMFS of patients with mSUVR ≤ 0.9 and patients with mSUVR > 0.9 was 30 months (95% CI, 13 to 46 months) and 11 months (95% CI, 5 to 17 months), respectively. Survival differences between the two groups were statistically significant in OS (p = 0.01), but not statistically significant in DMFS (p = 0.29).
Multivariate analysis was performed, in which age, performance status, stage, and use of chemotherapy during RT were considered in addition to the maxSUV and the cutoff mSUVR. As a continuous variable, the mSUVR and the maxSUVs of the primary tumor and the lymph node were not correlated with OS (Table 1). However, in categorical method, patients with mSUVR > 0.9 were associated with a 1.85-fold increase in the risk of death compared to those with mSUVR ≤ 0.9, and it is statistically significant (hazard ratio [HR] = 1.85; p = 0.05). Another significant association was observed between performance status and OS (HR = 2.22; p = 0.05). Details of the multivariate models are found in Table 2. Combining the two stratification variables, the 2-year OS for patients were as follows: with both good performance (Eastern Cooperative Oncology Group [ECOG] ≤ 1) and mSUVR ≤ 0.9, 71%; with either poor performance (ECOG > 1) or mSUVR > 0.9, 23% (p = 0.04) (Fig. 2). Only 4 patients had poor performance status and disease with high mSUVR, and all died before 2 years from the end of RT. The statistical difference among 3 groups, divided by the number of risk factors (poor performance and high mSUVR), was significant in OS (p = 0.001). There was no difference between two groups divided by mSUV = 0.9, in patient characteristics (Table 3), and the median maxSUV for disease was not different between two groups (10.3 for each ones).
Discussion and ConclusionIn the present study, we found that pretreatment mSUVR is a strong and independent prognostic predictor for OS in patients treated with definitive RT for lymph node positive NSCLC. When clinical cutoff value was used, patients with mSUVR ≤ 0.9 had longer OS than patients with mSUVR > 0.9. These findings are consistent with previously published data, advocating the prognostic role of FDG PET [16,20,21]. However, this study is the first to demonstrate that a metabolic activity of lymph node has a prognostic value, with adoption of the parameter of maxSUV ratio between primary tumor and lymph node.
The maximal SUV of primary tumor on FDG PET has significant predictive value, and it can demonstrate the metabolic activity and aggressiveness of primary tumor. But it has some limitation because tumor cells usually show heterogeneity with progression, and especially, with metastasis. If there is no evidence of gross distant metastasis and the primary lesions are successfully treated like in this study, the prognosis of the patients is critically affected by characteristics of metastatic cells. The metabolic activity of metastatic lymph node can be a surrogate indicator for characteristics of metastatic cells.
For this purpose, we adopted two parameters for metabolic activity of lymph nodes; maxSUVLN and mSUVR. As lymph nodes may show variable metabolic activity due to benign reactive activation due to cancer and inflammation, mSUVR may be a better parameter than maxSUVLN. With similar concept, mSUVR has been suggested as a diagnostic parameter for lymph node metastasis. In a retrospective review of a prospective database, Cerfolio and Bryant [19] evaluated the clinical utility of the ratio of the maxSUV of the mediastinal lymph node to the maxSUV of the primary tumor in patients with NSCLC. The median ratio of malignant lymph nodes was 0.58, and the authors found that a ratio of 0.56 or greater was predictive of lymph node malignancy with 94% sensitivity and 72% specificity.
Metabolic activity of NSCLC is known to have a correlation with tumor aggressiveness, due to its intrinsic relation with tumor doubling time [22] and proliferation rates [23,24]. How then can it be explained that the metabolic ratio of the lymph node to the primary tumor has a prognostic value in NSCLC? First, as mentioned above, the mSUVR may be a predictor of nodal malignancy. The routine mediastinal biopsy is not mandatory for every lung cancer patients in our hospital. Although invasive techniques provide additional information in microscopic examination of cancer cells, recent innovation of non-invasive methods such as FDG PET gives insights to physicians to the consideration of 'risk-benefit ratio'. Second, we regarded the mSUVR as a relative activity of nodal disease and a capacity of disease for dissemination out of the nodal station. Thus, patients with a high mSUVR were expected to have a high risk of out-field nodal recurrence, an isolated recurrence outside of the radiation field. However, only 1 patient (6%) with mSUVR > 0.9 and 3 patients (6%) in the group with mSUVR ≤ 0.9 developed out-field nodal recurrence. These rates seem to be reasonable when considering a recent report from Memorial Sloan-Kettering Cancer Center, in which only 9% of patients had suffered from unexpected nodal recurrence without elective nodal RT [25]. Also, there was no difference in distant failure between the two groups, and we found no statistically significant difference in locoregional failure-free survival either (p = 0.26, data not shown). Also, mSUVR for patients with out-field nodal recurrence were 0.44, 0.47, 0.53, and 1. Thus a high mSUVR does not provide a clue to out-field nodal recurrence, but it may be associated with intrinsic aggressiveness of disease.
Our study has several limitations. First, it suffers from a retrospective nature. There may be a selection bias, and there was a small variation in systemic therapy as well. The most significant critique of this study may come from the differences of total cycles and/or timing of combined chemo-regimen. But, 43 of 68 patients were treated with concurrent chemoradiotherapy, and platinum-based multi-drug regimens were infused weekly, such as docetaxel with cisplatin or gemcitabine with carboplatin. It is noteworthy that the use of the concurrent chemoradiotherapy did not prove to be a significant factor and the mSUVR was a stronger prognostic factor for OS. Second, histological confirmation of the metastatic sites was not performed in patients. And this may be weak point of the study for it is well-known that FDG accumulate in benign condition, such as granulomatous lymphadenitis. Third, our study included 6 patients with N1 disease. For evaluation of N1 status in NSCLC patients, FDG PET/CT has limited predictive value [26]. But, metabolic exam of N1 patients showed hypermetabolic activity (13.4 and 21.9 for 1-cm sized nodes), though the short axis length of lymph node of ranged from 1 to 2.1 cm.
An implication of our findings is that patients with high mSUVR might be candidates for more intensive therapy, such as chemoradiotherapy based on novel agents. Also, as high mSUVR is associated with a high risk of death, a period of close examination for disease is mandatory, especially when patients belong to a very high risk subgroup identified by the combination of high mSUVR and poor performance status.
In conclusion, in NSCLC, pretreatment mSUVR is prognostic of OS in patients treated with definitive RT for lymph node positive disease. This FDG PET parameter remained prognostic of OS when controlling for age, performance, stage, and use of chemotherapy. Although prospective studies with large numbers of cases or with longer follow-up are warranted for confirmation, we have demonstrated that the mSUVR is important aid for use in treatment decisions for patients with NSCLC.
AcknowledgmentsThis work was supported by a grant No. 03-2011-0270 from the Seoul National University Hospital Research Fund, a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111098 and A120313), and National R&D Program through the Dongnam Institute of Radiological & Medical Sciences funded by the Ministry of Education, Science and Technology (No. 50595-2012).
References1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108, PMID: 15761078.
2. Lee HK, Kwon HC, Lee SY, Kim JS. Treatment outcome of locally advanced non-small cell lung cancer. J Korean Soc Ther Radiol Oncol 2006;24:237–242.
3. Graziano SL. Non-small cell lung cancer: clinical value of new biological predictors. Lung Cancer 1997;17(Suppl 1):S37–S58, PMID: 9213302.
4. Kanters SD, Lammers JW, Voest EE. Molecular and biological factors in the prognosis of non-small cell lung cancer. Eur Respir J 1995;8:1389–1397, PMID: 7489807.
5. MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by PET in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:287–293, PMID: 11380213.
6. Vesselle H, Pugsley JM, Vallieres E, Wood DE. The impact of fluorodeoxyglucose F-18 positron-emission tomography on the surgical staging of non-small cell lung cancer. J Thorac Cardiovasc Surg 2002;124:511–519, PMID: 12202868.
7. Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879–892, PMID: 14644890.
8. So Y, Chung JK, Jeong JM, Lee DS, Lee MC. Usefulness of additional delayed regional F-18 fluorodeoxy-glucose positron emission tomography in the lymph node staging of non-small cell lung cancer patients. Cancer Res Treat 2005;37:114–121, PMID: 19956490.
9. Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer 2005;49:95–108, PMID: 15949595.
10. Hicks RJ, Mac Manus MP, Matthews JP, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys 2004;60:412–418, PMID: 15380574.
11. Sura S, Greco C, Gelblum D, Yorke ED, Jackson A, Rosenzweig KE. (18)F-fluorodeoxyglucose positron emission tomography-based assessment of local failure patterns in non-small-cell lung cancer treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1397–1402, PMID: 18374225.
12. Vaidya M, Creach KM, Frye J, Dehdashti F, Bradley JD, El Naqa I. Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother Oncol 2012;102:239–245, PMID: 22098794.
13. Ohno Y, Koyama H, Yoshikawa T, et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 2012;198:75–82, PMID: 22194481.
14. Gregory DL, Hicks RJ, Hogg A, et al. Effect of PET/CT on management of patients with non-small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med 2012;53:1007–1015, PMID: 22677701.
15. Bradley J, Thorstad WL, Mutic S, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:78–86, PMID: 15093902.
16. Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12, PMID: 18166834.
17. Vesselle H, Freeman JD, Wiens L, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 2007;13:3255–3263, PMID: 17545531.
18. Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE 2nd, Patz EF Jr. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol 2008;26:1459–1464, PMID: 18349396.
19. Cerfolio RJ, Bryant AS. Ratio of the maximum standardized uptake value on FDG-PET of the mediastinal (N2) lymph nodes to the primary tumor may be a universal predictor of nodal malignancy in patients with nonsmall-cell lung cancer. Ann Thorac Surg 2007;83:1826–1829, PMID: 17462407.
20. Borst GR, Belderbos JS, Boellaard R, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer 2005;41:1533–1541, PMID: 15953716.
21. Sasaki R, Komaki R, Macapinlac H, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005;23:1136–1143, PMID: 15718309.
22. Duhaylongsod FG, Lowe VJ, Patz EF Jr, Vaughn AL, Coleman RE, Wolfe WG. Detection of primary and recurrent lung cancer by means of F-18 fluorodeoxyglucose positron emission tomography (FDG PET). J Thorac Cardiovasc Surg 1995;110:130–139, PMID: 7609536.
23. Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 2000;6:3837–3844, PMID: 11051227.
24. Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3'deoxy-3'-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002;8:3315–3323, PMID: 12429617.
25. Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non-small-cell lung cancer. J Clin Oncol 2007;25:5557–5561, PMID: 17984185.
26. Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited predictive value of dual-time-point F-18 FDG PET/CT for evaluation of pathologic N1 status in NSCLC patients. Clin Nucl Med 2011;36:434–439, PMID: 21552019.
Fig. 1Cutoff of maximal SUV ratio (mSUVR) versus log-rank p-value for overall survival (OS, A) and distant metastasis-free survival (DMFS, B) of eligible patients. 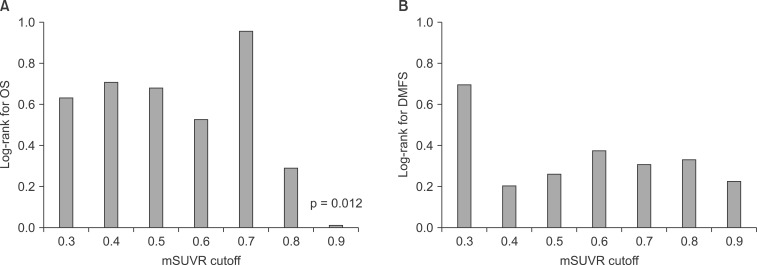
Fig. 2Influence of performance status and maximal SUV ratio (mSUVR) on overall survival (OS) of 68 patients with non-small-cell lung cancer after definitive radiotherapy. ECOG, Eastern Cooperative Oncology Group. 
Table 1Multivariate analysis of different prognostic factors for OS in patients with node positive non-small-cell lung cancer treated with definitive radiotherapy (n = 68) 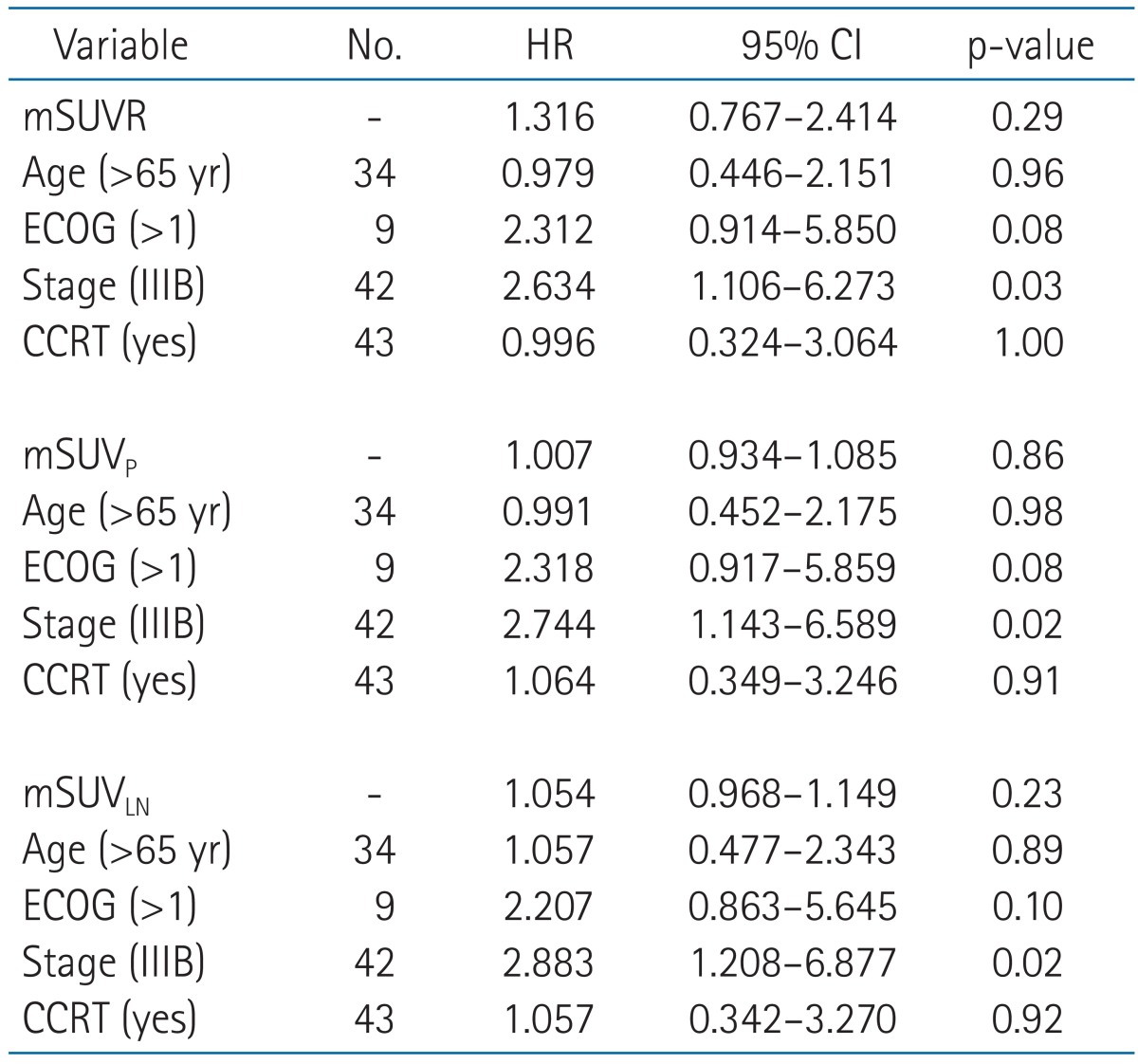 The values of SUV-related parameters were used for model formation as continuous variables. OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; CCRT, concurrent chemotherapy and radiotherapy; mSUVR, maximal SUV ratio; mSUVP, maximal SUV of primary tumor; mSUVLN, maximal SUV of lymph node. Table 2Multivariate analysis of different prognostic factors in patients with node positive non-small cell lung cancer treated with definitive radiotherapy (n=68) 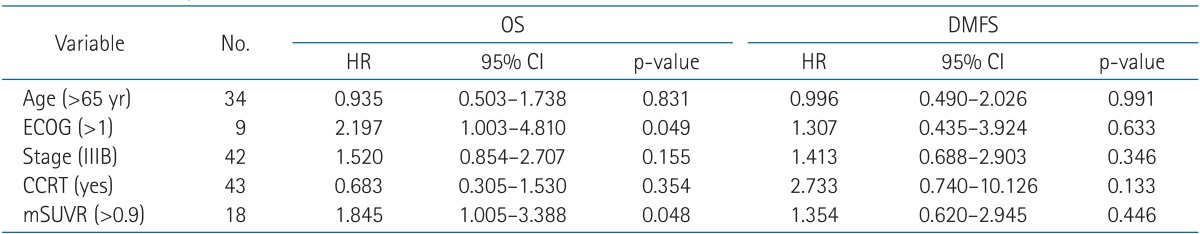
|
|
||||||||||||||||||||||||||||||||||||

 |
 |